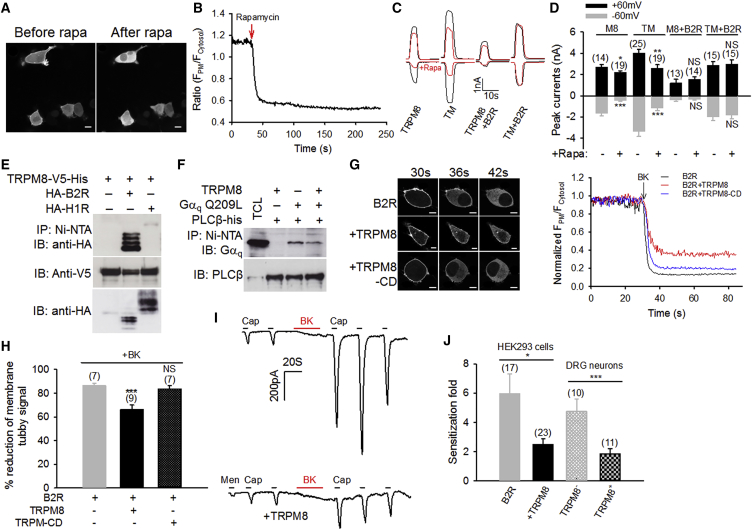
Figure 7.
TRPM8 Is Insensitive to PIP2 Depletion in the Presence of B2R
(A) Translocation of Tubby-R332H-cYFP induced by rapamycin (1 μM) in HEK293 cells co-expressing mRFP-FKBP-5-ptase domain and PM-FRB-CFP. Scale bars, 10 μM.
(B) Real-time quantification of membrane fluorescence (FPM) relative to cytosol fluorescence (FCytosol) in the top left cell from (A).
(C) Example of whole-cell inward and outward currents elicited by menthol in HEK293 cells co-expressing mRFP-FKBP-5-ptase and PM-FRB-CFP together with either TRPM8 and B2R or TRPM8-TM and B2R. Red traces indicate cells pretreated with rapamycin (Rapa, 1 μM, 1 min).
(D) Collective results from experiments similar to those in (C).
(E) Binding of TRPM8 to B2R, but not to H1R, in a nickel-beads (Ni-NTA) pull-down assay performed on HEK293 cell lysate expressing the proteins as indicated.
(F) Binding between PLCβ-his (6×) and Gαq Q209L is reduced in the presence of TRPM8 in a nickel beads pull-down assay. Lane 1 indicates total cell lysate (TCL).
(G) Translocation of Tubby-R332H-cYFP at different time points after the addition of BK (1 μM) in HEK293 cells co-expressing B2R or together with TRPM8 or C-terminal deleted (TRPM8-CD). Scale bars, 10 μM. On the right, real-time quantification of membrane Tubby fluorescence is indicated relative to cytosol fluorescence from the cells in (G).
(H) Summary of results similar to those in (G).
(I) Example inward currents evoked by capsaicin (Cap, 100 nM, 5 s) in HEK293 cells expressing TRPV1 and B2R or together with TRPM8 before and after BK treatment (1 μM, red). TRPM8 co-expression is indicated by currents elicited by menthol (Men, 200 μM).
(J) Summary of TRPV1 sensitization fold induced by BK in experiments similar to those in (I). BK-induced sensitization of TRPV1 was also significantly different between TRPM8+ and TRPM8− DRG neurons indicated by responses to menthol.
All error bars represent mean ± SEM. ∗∗p < 0.01; ∗∗p < 0.05; ∗∗∗p < 0.001; NS, not significant. See also Figure S4.