Figure 1.
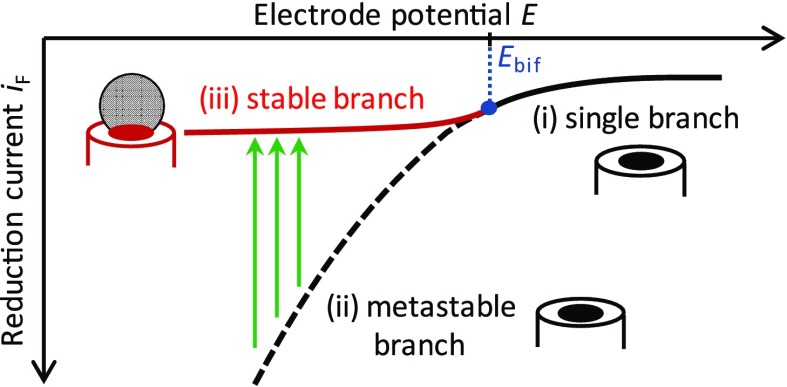
Bifurcation in the nanoelectrode–nanobubble system. Consider nanobubbles generated by a reduction reaction, for example, H+ reduction. The relation between the reduction current, iF, and the electrode potential, E, exhibits three distinct regimes: (i) For E higher than a critical bifurcation potential, Ebif (shown in blue), the formation of nanobubbles is thermodynamically unfavorable. In this potential range, no nanobubble is formed at the electrode, and the iF – E relation is single-valued (single branch, solid black line). (ii) At potentials E < Ebif, the stable dynamical state exhibits a nanobubble that blocks most of the electrode surface, thus limiting the magnitude of the reduction current (stable branch, solid red line). If nucleation of the nanobubble was instantaneous, the iF – E relation would transition smoothly between branches (i) and (ii). (iii) Nucleation of the nanobubble takes place at a finite rate. When the potential is switched or swept to E < Ebif, there is a time interval in which no nanobubble is present at the electrode. The reduction current is then larger than that in the stable branch at the same potential since the entire electrode surface participates in the reduction reaction (metastable branch, dashed black line). This results in a higher degree of gas supersaturation near the electrode, which in turns facilitates nucleation. Once nucleation occurs, the system switches from the metastable to the stable branch (green arrows), where it remains until the potential is increased to E > Ebif. In practice, the nanobubble nucleation rate is immeasurably slow near E = Ebif but increases rapidly with increasing current, thus permitting the experimental observation of nanobubbles.