Table 2.
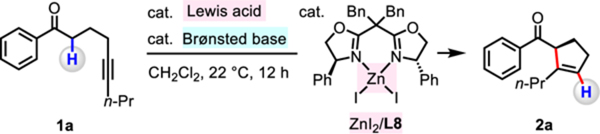 | |||||
|---|---|---|---|---|---|
| entry | Lewis acid (mol%) | Brønsted base (mol%) | Znl2/L8 (mol%) | yield of 2a (%) | er |
| 1 | B(C6F5)3 (10) | NET3 (20) | 10 | >95 | 97:3 |
| 2 | B(C6F5)3 (10) | DBU (20) | 10 | 0 | ND |
| 3 | B(C6F5)3 (10) | PMP (20) | 10 | >95 | 97:3 |
| 4 | B(C6F5)3 (7.5) | PMP (15) | 7.5 | 90 | 97:3 |
| 5c | B(C6F5)3 (7.5) | PMP (15) | 7.5 | >95 | 97:3 |
| 6c | B(C6F5)3 (5.0) | PMP (10) | 5.0 | 92 | 97:3 |
| 7 | B(C6F5)3 (5.0 | none | 5.0 | 0 | ND |
| 8 | none | PMP (10) | 5.0 | 0 | ND |
| 9 | B(C6F5)3 (5.0) | PMP (10) | 0 | 0 | ND |
| 10 | BF3·OEt2 (5.0) | PMP (10) | 5.0 | 0 | ND |
| 11 | BPh3 (5.0) | PMP (10) | 5.0 | 0 | ND |
Conditions: 1-phenylnon-5-yn-1-one (1a, 0.2 mmol), organoborane, Brønsted base, ZnI2/L8 complex, CH2Cl2 (1.0 mL), under N2, 22 °C, 12 h.
Yield was determined by 1H NMR analysis of unpurified reaction mixtures with mesitylene as the internal standard. The er values were determined by HPLC analysis of the purified product.
The reaction mixture was allowed to stir at 40 °C.
