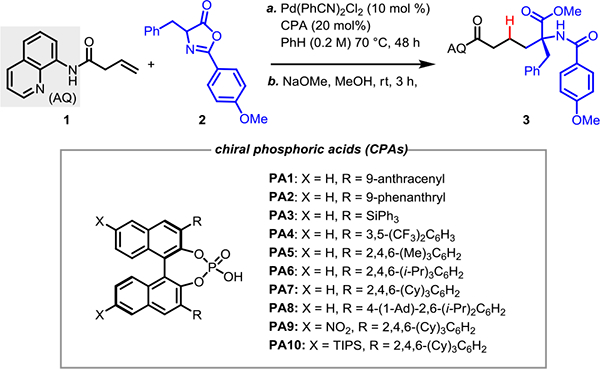
Table 1.
Optimization of the Reaction Conditions[a]
| Entry | Promoter (20%) | Yield[b](%) | Enantiomeric Ratio (er)[c] |
|---|---|---|---|
| 1 | - | 12 | 50:50 |
| 2 | PA1 | 74 | 83:17 |
| 3 | PA2 | 69 | 75:25 |
| 4 | PA3 | 29 | 55:45 |
| 5 | PA4 | 69 | 65:35 |
| 6 | PA5 | 73 | 80:20 |
| 7 | PA6 | 72 | 86:14 |
| 8 | PA7 | 90 | 91:9 |
| 9 | PA8 | 79 | 87:13 |
| 10[d] | PA7 | 75 | 92:8 |
| 11[d, e] | PA7 | 74 | 93:7 |
| 12 [d, e] | PA9 | 65 | 91:9 |
| 13 [d, e] | PA10 | 71 | 94:6 |
Reaction conditions: 1a (0.05 mmol), 2a (3.0 equiv), Pd(PhCN)2Cl2 (10 mol %), ligand (20 mol%), anhydrous benzene (0.2 M), 70 °C, 2 days.
Isolated yield.
Enantiomeric ratio determined by chiral SFC.
At 0.1 M concentration.
At 55 °C, 4 days.