Abstract
Background:
The mortality of cardiovascular disease is constantly rising, and novel biomarkers help us predict residual risk. This study aimed to evaluate the predictive value of serum homocysteine (HCY) levels on prognosis in patients with ST-segment elevation myocardial infarction (STEMI).
Methods:
The 419 consecutive patients with STEMI, treated at one medical center, from March 2010 to December 2015 were retrospectively investigated. Peripheral blood samples were obtained within 24 h of admission and HCY concentrations were measured using an enzymatic cycling assay. The patients were divided into high HCY level (H-HCY) and low HCY level (L-HCY) groups. Short- and long-term outcomes were compared, as were age-based subgroups (patients aged 60 years and younger vs. those older than 60 years). Statistical analyses were mainly conducted by Student t-test, Chi-squared test, logistic regression, and Cox proportional-hazards regression.
Results:
The H-HCY group had more males (84.6% vs. 75.4%, P = 0.018), and a lower prevalence of diabetes (20.2% vs. 35.5%, P < 0.001), compared with the L-HCY group. During hospitalization, there were seven mortalities in the L-HCY group and 10 in the H-HCY group (3.3% vs. 4.8%, P = 0.440). During the median follow-up period of 35.8 (26.9–46.1) months, 33 (16.2%) patients in the L-HCY group and 48 (24.2%) in the H-HCY group experienced major adverse cardiovascular and cerebrovascular events (MACCE) (P = 0.120). History of hypertension (hazard ratio [HR]: 1.881, 95% confidence interval [CI]: 1.178–3.005, P = 0.008) and higher Killip class (HR: 1.923, 95% CI: 1.419–2.607, P < 0.001), but not HCY levels (HR: 1.007, 95% CI: 0.987–1.027, P = 0.507), were significantly associated with long-term outcomes. However, the subgroup analysis indicated that in older patients, HCY levels were significantly associated with long-term outcomes (HR: 1.036, 95% CI: 1.011–1.062, P = 0.005).
Conclusion:
Serum HCY levels did not independently predict in-hospital or long-term outcomes in patients with STEMI; however, among elderly patients with STEMI, this study revealed a risk profile for late outcomes that incorporated HCY level.
Keywords: Homocysteine, Acute ST-segment elevation myocardial infarction, Percutaneous coronary intervention, Clinical outcome
Introduction
Cardiovascular diseases are currently the major causes of mortality worldwide.[1,2] In China, the prevalence and mortality of cardiovascular diseases are constantly rising. While traditional risk factors are the main therapeutic targets for risk stratification, novel biomarkers may further predict residual risk, and therefore improve the effectiveness of primary and secondary prevention.[3,4]
Homocysteine (HCY), a non-essential sulfur-containing amino acid, is an intermediate metabolite of methionine. Basic experiments indicated that HCY might be a risk factor for atherosclerosis.[5] Furthermore, there is clinical evidence that higher HCY levels may be associated with poor outcomes in patients with stable angina, stroke, and peripheral artery disease.[6,7] Nevertheless, the predictive value of serum HCY levels in patients with ST-segment elevation myocardial infarction (STEMI), which is caused by acute coronary occlusion, remains uncertain. This study was to compare HCY levels and short- and long-term outcomes in patients with STEMI.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University. Given the retrospective nature of this study, written informed consent was waived.
Study population
The study cohort included 419 consecutive patients with STEMI who presented at Xuanwu Hospital, Capital Medical University from March 2010 to December 2015. STEMI was defined according to the presence of at least two of the following three criteria: (1) periods of prolonged chest pain (≥20 min); (2) ST-segment elevation ≥2 mm in at least two contiguous precordial leads, or ST-segment elevation ≥1 mm in at least two inferior leads, or new left bundle branch block[8]; and (3) elevation of a myocardial biomarker (cardiac troponin [cTn] or creatine kinase MB/isoenzyme of creatine kinase [CK-MB]) >99th percentile or two times the normal value. Patients were excluded if met the following criteria: (1) taking a folic acid supplement; (2) with a prior history of STEMI; (3) with a prior history of coronary revascularization; or (4) acute or chronic inflammatory disease.
Baseline information (clinical characteristics, laboratory results, and angiography) were collected by interviewing patients and medical record reviews. Patients were classified into two groups according to their serum HCY levels in relation to the median level (14.4 μmol/L). For the subgroup analysis, patients were divided into two subgroups according to age (≤60 years and >60 years).
Coronary angiography
Diagnostic coronary angiography was performed using 5 to 6 French Judkins catheters (Cordis Corp., Miami Lakes, Florida, USA) and either a radial or femoral approach.[9] Performance of percutaneous coronary intervention (PCI) was determined by operators. All stenosis were evaluated visually and the Gensini score was determined.[10] Single vessel disease was defined as stenosis of at least 50% in one major epicardial coronary artery, while multi-vessel disease was defined as stenosis of at least 50% in ≥ two major epicardial coronary arteries.
Biochemical measurements
Peripheral blood samples were obtained within 24 h of hospital admission. After coagulation at room temperature for 30 min, the samples were centrifuged at 3108 × g for 5 min and the serum was aspirated. Serum HCY concentrations were measured by enzymatic cycling assay according to the kit instructions (Maccura Biotechnology Co., Ltd., Chengdu, China) with the Hitachi Automatic Analyzer 7600–210 (Hitachi, Tokyo, Japan). Serum concentrations of creatinine, uric acid, hemoglobin glycosylated hemoglobin (HbA1c), total cholesterol, high density lipoprotein (HDL)-cholesterol, and low density lipoprotein (LDL)-cholesterol were assessed using standard laboratory methods.
In-hospital death and long-term outcomes
The in-hospital mortality, defined as all-cause death, was compared between groups. Patients were followed up via telephone calls at six and 12 months, and then annually. The primary endpoint was defined as major adverse cardiovascular and cerebrovascular events (MACCE), including all-cause death, nonfatal myocardial infarction (MI), stroke, heart failure, and revascularization.
Statistical analysis
Continuous variables are shown as mean ± standard deviation (SD) and compared using a two-tailed Student t-test, while medians (Q1, Q3) and Mann–Whitney U tests were used for non-normally distributed variables. Categorical variables are shown as frequency (group percentage) and between-group comparisons were made using the Pearson Chi-square or Fisher exact test. Serum HCY was first considered as a categorical variable divided into two groups (H-HCY and L-HCY), and later as a continuous value to examine the relationship with end points. Bivariate correlations were analyzed using the Pearson correlation model. Binary logistic regression was used for multivariate analyses to determine predictors of in-hospital events. Kaplan–Meier estimates were used to estimate survival curves, and the log-rank test to test between-group differences. Cox proportional-hazards regression models were used to examine the relationship between HCY levels and different end points, after adjustment for multiple clinical and angiographic covariates significantly associated with each end point during univariate analysis. Hazard ratios (HRs) were reported with corresponding 95% confidence intervals (CIs). The respective predictive cut-off values were constructed according to the receivers operating characteristic curve for HCY for discrimination between surviving and MACCE. The areas under the curve were compared using the Hanley and McNeil method. Statistical analyses were performed using the Statistical Package for Social Sciences software version 19.0 (SPSS Inc., Chicago, IL, USA). A two-sided P < 0.05 was considered statistical significance.
Results
Baseline characteristics
A total of 419 consecutive patients were enrolled. The mean age was 62.0 ± 12.2 years and 336 (80.2%) were males. The baseline clinical and angiographic characteristics of the two groups are presented in Table 1. The H-HCY group had more male patients (84.6% vs. 75.4%, P = 0.018) and fewer patients with diabetes (20.2% vs. 35.5%, P < 0.001), compared with the L-HCY group. The levels of white blood cell (WBC), creatinine, and uric acid (UA) were higher in the H-HCY group, compared with the L-HCY group (all P < 0.001). There were no significant differences in prior medication use between L-HCY and H-HCY groups, with the exception of calcium channel blockers (28.0% vs. 16.8%, P = 0.005). The numbers of diseased vessels and Gensini scores were similar between the two groups.
Table 1.
Baseline characteristics of patients with ST-segment elevation myocardial infarction according to HCY levels.

Correlation between serum HCY and biochemistry indicators
Serum HCY levels were positively correlated with WBC count (r = 0.146, P = 0.003), creatinine (r = 0.254, P < 0.001) and UA level (r = 0.278, P < 0.001).
In-hospital outcomes
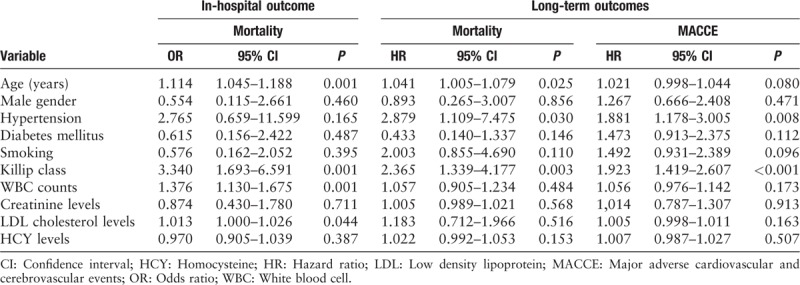
Seven patients died in the L-HCY group and 10 in the H-HCY group (3.3% vs. 4.8%, P = 0.440). After adjusting for general conditions and blood biochemistry indicators, serum HCY level (OR: 0.970, 95% CI: 0.905–1.039, P = 0.387) was not significantly associated with mortality. Age (OR: 1.114, 95% CI: 1.045–1.188, P = 0.001), Killip class (OR: 3.340, 95% CI: 1.693–6.591, P = 0.001), WBC counts (OR: 1.376, 95% CI: 1.130–1.675, P = 0.001) and creatinine levels (OR: 1.013, 95% CI: 1.000–1.026, P = 0.044) were independent predictors for in-hospital mortality [Table 2].
Table 2.
Multivariate logistic and Cox regression analyses on in-hospital mortality and long-term outcomes.
Long-term outcomes
Follow-up information was available for 324 (80.6%) patients; 78 patients were lost to follow-up. Rates of lost follow-up were almost equal between L-HCY and H-HCY groups in overall population (21.6% vs. 17.2%, P = 0.265) as well as elder group (15.9% vs. 15.0%, P = 0.852). During the median follow-up period of 35.8 (26.9, 46.1) months, 81 (20.2%) patients experienced MACCE, including 33 in L-HCY group and 48 in H-HCY group. The unadjusted Kaplan–Meier estimates of MACCE at 7 years were comparable between the two groups (P=0.120) [Figure 1].
Figure 1.
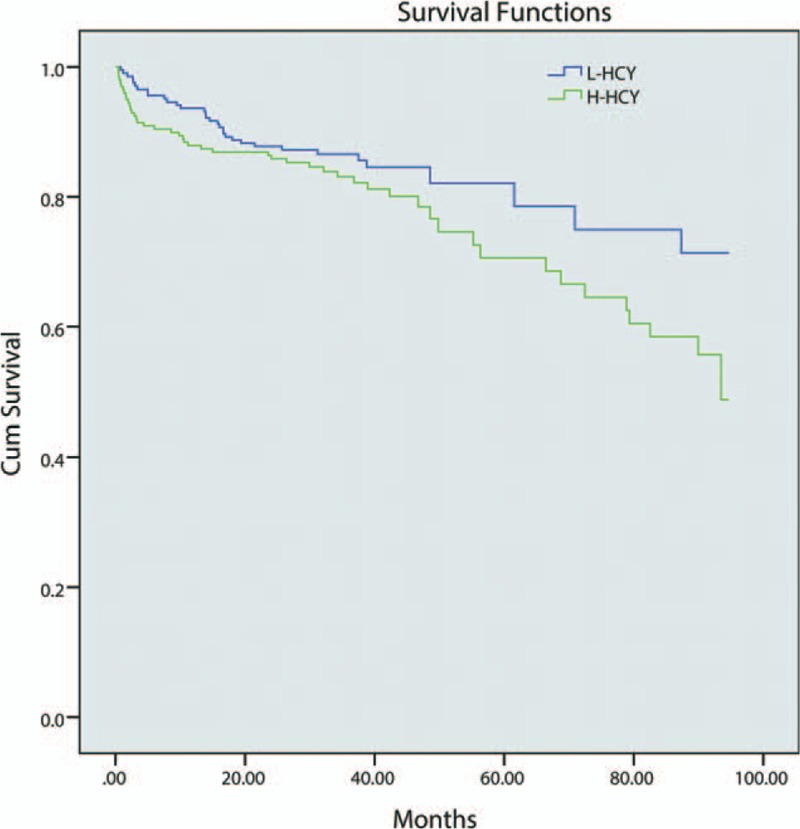
Kaplan–Meier survival rate curve for MACCE. The unadjusted Kaplan–Meier estimates of MACCE at 7 years were comparable between two groups (P = 0.120). H-HCY: High homocysteine level; L-HCY: Low homocysteine level; MACCE: Major adverse cardiovascular and cerebrovascular events.
Cox multivariate models for long-term outcomes
The independent predictive value of HCY level on long-term mortality (HR: 1.022, 95% CI: 0.992–1.053, P = 0.153) and MACCE (HR: 1.007, 95% CI: 0.987–1.027, P = 0.507) was not statistically significant. Age (HR: 1.041, 95% CI: 1.005–1.079, P = 0.025), history of hypertension (HR: 2.879, 95% CI: 1.109–7.475, P = 0.030) and Killip class (HR: 2.365, 95% CI: 1.339–4.177, P = 0.003) retained statistical significance for long-term mortality [Table 2]. Meanwhile, hypertension (HR: 1.881, 95% CI: 1.178–3.005, P = 0.008) and Killip class (HR: 1.923, 95% CI: 1.419–2.607, P < 0.001) were independent predictors of long-term MACCE.
Subgroup analysis
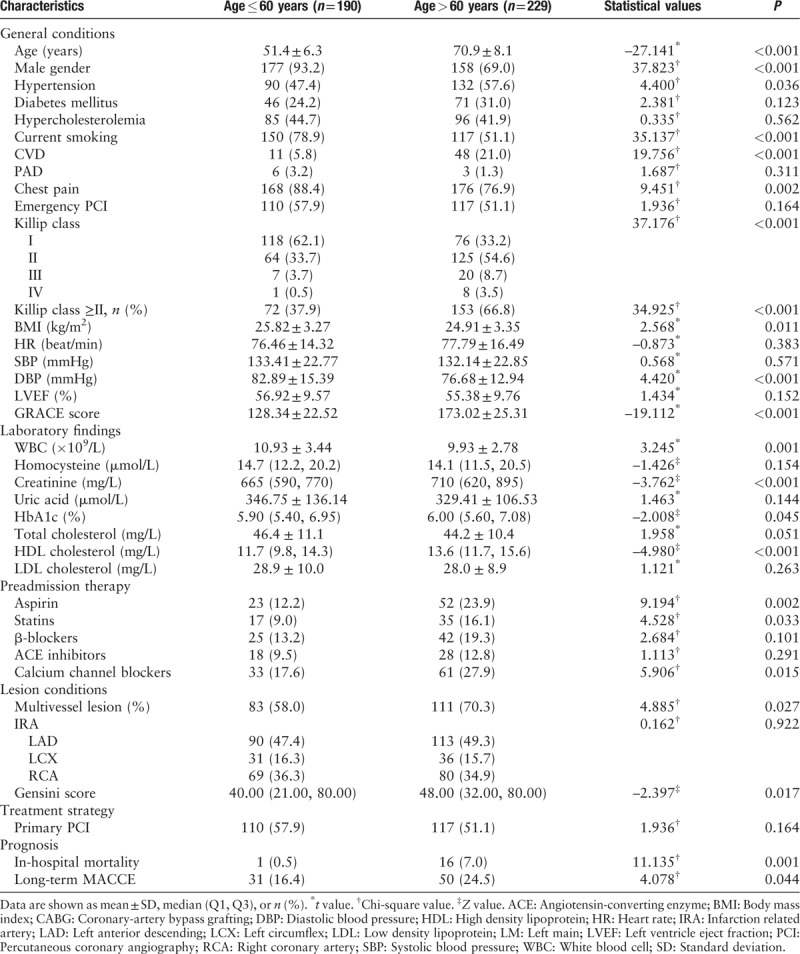
All subjects were classified according to age as either younger (age ≤60 years) or older (age >60 years) groups and their clinical characteristics were compared [Table 3]. The younger group included more males (93.2% vs. 69.0%, P < 0.001) and smokers (78.9% vs. 51.1%, P < 0.001). In contrast, the older group had a worse average Killip classification (66.8 vs. 37.9%, P < 0.001), higher mean GRACE score (128.34 ± 22.52 vs. 173.02 ± 25.31, P < 0.001) and more multivessel disease (70.3% vs. 58.0%, P = 0.027). After following a similar treatment strategy, one patient died in the younger group while 16 patients died in older group during hospitalization (0.5% vs. 7.0%, P = 0.001). During follow-up, 31 patients in the younger group and 50 in the elder group suffered MACCE (16.4% vs. 24.5%, P = 0.044).
Table 3.
Baseline characteristics of patients with ST-segment elevation myocardial infarction according to age.
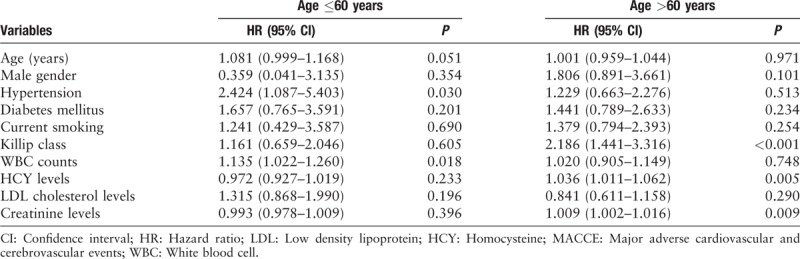
After adjustment for confounding factors in the multivariate Cox regression analysis, hypertension (HR: 2.424, 95% CI: 1.087–5.403, P = 0.030) and WBC count (HR: 1.135, 95% CI: 1.022–1.260, P = 0.0181) remained independent predictors for the younger group, while Killip class (HR: 2.186, 95% CI: 1.441–3.316, P < 0.001), HCY level (HR: 1.036, 95% CI: 1.011–1.062, P = 0.005) and creatinine level (HR: 1.009, 95% CI: 1.002–1.016, P = 0.009) retained statistical significance for the older group [Table 4].
Table 4.
Multivariate Cox regression analysis on long-term MACCE.
Diagnostic power of HCY for long-term MACCE in the older group
The area under the receiver operating characteristics curve was 0.662 (0.579, 0.746, P < 0.001) [Figure 2]. The predictive cut-off value of HCY for MACCE was 14.05 μmol/L (sensitivity: 0.740; specificity: 0.564).
Figure 2.
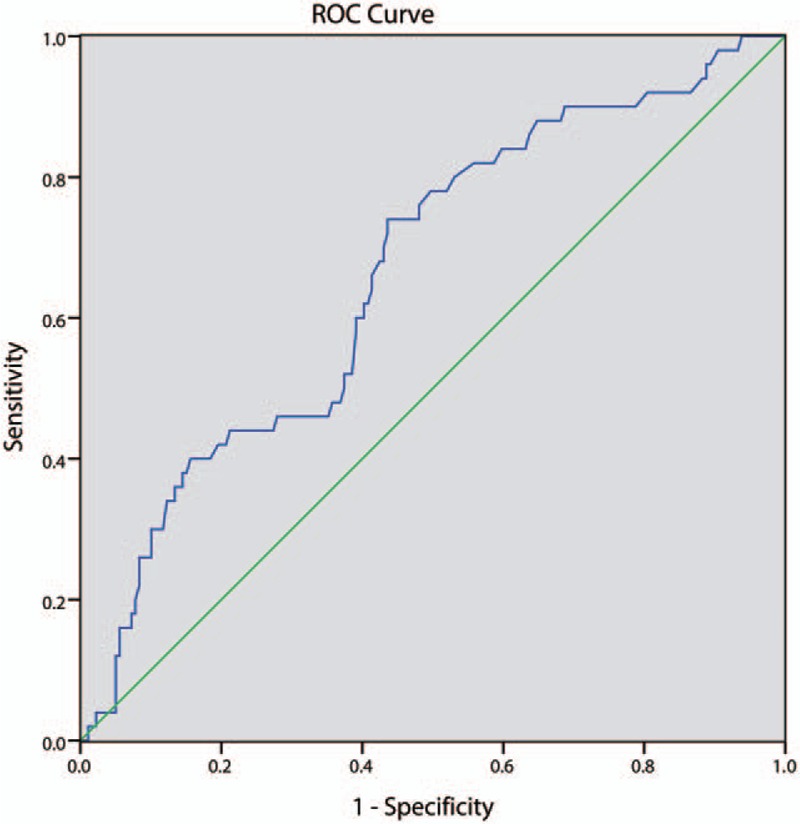
The receivers operating characteristic curve for homocysteine in subgroup with age >60 years. Area under the receiver operating characteristics curve was 0.662 (0.579, 0.746; P < 0.001). The predictive cut-off value of homocysteine level for MACCE was 14.05 μmol/L (sensitivity: 0.740 and specificity: 0.564). MACCE: Major adverse cardiovascular and cerebrovascular events.
Discussion
There were no statistically significant associations between serum HCY levels and short- and long-term outcomes among all patients; however, among older patients, HCY independently predicted prognosis. Traditional risk factors, such as age, hypertension, and Killip class, remain the predominant predictors for outcomes in patients with STEMI.
HCY is formed during metabolism of methionine, and its recycling is mediated by vitamin B6, B12, or folic acid. Dietary deficiency of the aforementioned vitamins, genetic abnormalities including CBS and MTHFR mutations, and kidney failure are the main causes of elevated serum HCY levels, termed homocysteinemia.
It is widely accepted that elevated plasma HCY levels are associated with increased adverse cardiovascular events, independent of other factors.[11,12] Evidence from case-control and prospective studies suggested a graded and independent association between HCY levels and prognosis in patients with coronary artery disease (CAD). Nygard et al[13] reported that HCY levels were strong predictors of long-term mortality in 587 patients with angiographically confirmed CAD, after a median follow-up period of 4.6 years.
Besides its heavy burden to health, there is inadequate or conflicting evidence of the prognostic value of HCY in patients with acute coronary syndrome (ACS).[14,15] Omland et al[16] firstly investigated the possible prognostic value of HCY on survival in 579 patients with acute coronary syndrome after a median follow-up of 628 days. While Foussas et al[17] suggested HCY levels on admission were not an independent predictor of long-term mortality in patients with ACS.
Meanwhile, elevated serum HCY level was accompanied by the prevalence of clinical and subclinical cerebral injury, as well as long-term overall mortality and stroke recurrence.[18] However, the efficiency of HCY lowing therapy in stroke prevention warrant further investigation.[19]
Nevertheless, due to the lack of relevant research, the possibility for HCY level in predicting outcomes of STEMI remains unclear.
Above all, the pathophysiological mechanism of serum HCY-mediated vascular dysfunction and STEMI procedure triggering was not in full accord.
STEMI is characterized as rupture of an atherosclerotic plaque, alteration in coronary vasomotor tone, platelet aggregation, and an active thrombotic procedure, which lead to interruption of blood flow and MI.[20] Pathological studies and intracoronary imaging showed that the occurrence of STEMI procedure directly associated with vulnerable plaques[21,22] and systemic low-grade inflammation.[23]
The mechanisms that elevated HCY impairs vascular function are not definitely known. Several potential mechanisms consist of endothelial dysfunction,[24] oxidative stress,[25] activation of inflammation,[26] proliferation of smooth-muscle, increased adhesion of monocytes to endothelial cells[27] and platelet dysfunction.[28,29]
During hospitalization, the critical risk factor of mortality was the infarct location and proportion. STEMI triggers a systemic acute-phase response, in which neutrophils and monocytes/macrophages attacks the infarcted myocardial.[23] Besides, systemic inflammation that manifested as elevated WBC count, is associated with impaired microvascular reperfusion after PCI. Elder age and worse Killip classification also symbolize poorer cardiac function. HCY might affect the infarction indirectly by activation of inflammation, but the effectiveness of which was too limited to be revealed.
As for long-term management, secondary prevention of CAD serves as the pivotal role. Before discharge, all patients were advised for regular medication (antihypertensive, antiplatelet, and lipid-lowing therapy), smoking cessation, body weight control, adequate exercise, and regular visit. High HCY level only leads to mild elevation of cardiovascular risk, which turns even inadequate after active secondary prevention was carried out.
During subgroup analysis, we drew different risk profiles for younger and older patients. Both groups shared similar HCY levels, but compared with younger patients, older patients exhibited higher creatinine levels and worse Killip risk stratification. Besides following similar treatment strategies, older patients exhibited poorer prognosis during hospitalization and follow-up. HCY level was retained as an independent prognostic factor in older patients, in addition to high Killip class and creatinine level. Between-group differences in clinical characteristics and pathophysiology led to risk stratification.
On the one hand, elevated HCY levels were previously related to congestive heart failure in patients with STEMI, which was defined as Killip II or higher.[30] The predictive significance of HCY on long-term mortality and MACCE emerged following addition of worse cardiac function as baseline in the older group. HCY is naturally broken down to be excreted in the urine. Thus, renal dysfunction might lead to elevation of serum HCY during long-term follow-up, by which elevated HCY revealed its prognostic value in patients with STEMI.
Current studies are testing the possible benefits of HCY-lowering therapy. Currently, there is insufficient evidence to recommend treatment of elevated HCY levels with folic acid or other vitamins to prevent cardiovascular diseases, which indirectly supports our results. The NORVIT trial indicated that, despite effectively reducing HCY levels, folic acid and vitamin B did not lower the risk of MACCE in patients with acute MI; notably, this study actually showed a trend toward increased cardiovascular risk.[31] Folic acid supplementation as a primary prevention for MI also failed in a Chinese population.[32] In our previous research, HCY did not serve as an independent prognostic factor in a patient population with STEMI, not to mention its utility as a therapeutic target.
In the current study, age, a history of hypertension, higher Killip class, higher creatinine level, and WBC count were independently related with worse prognosis in patients with STEMI. Thus, risk stratification should be carried out according to these characteristics. Additionally, more attention should be paid to patients in treatment during hospitalization and long-term management.
This study provided important, complementary information to previous studies of the prognostic value of HCY levels in patients with stable coronary artery disease or among those without cardiac diseases. The usefulness of HCY might be overrated, and large, randomized, controlled trials are necessary to determine the prognostic value of serum HCY concentrations on risk profile modification for patients with STEMI.
Several limitations to the present study should be considered. This was a single-center, cohort study with unequal baseline characteristics, involving multiple operators. Meanwhile, secondary prevention medications, residual atherothrombotic burdens on non-culprit vessels, and cardiac rehabilitation are important determiners of long-term prognosis, and may emerge as confounders. Multivariate regression analysis might mitigate bias by adjusting confounding factors, but unmeasured indicators might leave room for residual bias. Secondly, our sample size and number of events was limited. Cohort studies with larger sample sizes and long-term follow-up will be much more valuable. Rate of lost follow-up in current study was relatively high, which might impact on reliability of results, to a certain extent. At the same time, a small sample size, low follow-up rate, and relatively small events might lead to over fitting. Moreover, serum HCY levels were measured once upon admission to the present study. Serum HCY concentrations were not obtained during follow-up; thus, we were not able to include this valuable information in our analysis. Finally, we did not include patients who took folic acid supplements, so the prognostic value of HCY level interventions remained unclear in the present study.
In conclusion, this study provided evidence that elevated HCY levels on admission did not indicate increased risk of hospital duration or long-term mortality and MACCE among all patients with STEMI we examined. For patients older than 60 years, HCY predicted long-term MACCE.
Funding
This study was supported by the grants from the National Natural Science Foundation of China (No. 81470491), Beijing Natural Science Foundation (No. 7192078), and Open Foundation from Beijing Key Laboratory of Hypertension Research (No. 2017GXY-KFKT-04).
Conflicts of interest
None.
Footnotes
How to cite this article: Si J, Li XW, Wang Y, Zhang YH, Wu QQ, Zhang LM, Zuo XB, Gao J, Li J. Relationship between serum homocysteine levels and long-term outcomes in patients with ST-segment elevation myocardial infarction. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000159
Jin Si and Xue-Wen Li contributed equally to this work.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran AE, Tzong KY, Forouzanfar MH, Rothy GA, Mensah GA, Ezzati M, et al. Variations in ischemic heart disease burden by age, country, and income: the global burden of diseases, injuries, and risk factors 2010 study. Glob Heart 2014; 9:91–99. doi: 10.1016/j.gheart.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SC, Amsterdam E, Balady GJ, Bonow RO, Fletcher GF, Froelicher V, et al. Prevention conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention. Circulation 2000; 101:111–116. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham heart study. Circulation 2012; 126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JH, McBride PE. Hyperhomocysteinemia and atherosclerotic vascular disease: pathophysiology, screening, and treatment. Arch Intern Med 1998; 158:1301–1306. doi: 10.1001/archinte.158.12.1301. [DOI] [PubMed] [Google Scholar]
- 6.Eikelboom JW, Lonn E, Genest J, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med 1999; 131:363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325:1202.doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 9.Lavi S, Rihal CS, Yang EH, Fassa AA, Elesber A, Lennon RJ, et al. The effect of drug eluting stents on cardiovascular events in patients with intermediate lesions and borderline fractional flow reserve. Catheter Cardiovasc Interv 2007; 70:525–531. doi: 10.1002/ccd.21154. [DOI] [PubMed] [Google Scholar]
- 10.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606.doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 11.Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA 1997; 277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 12.Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, et al. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 2011; 58:1025–1033. doi: 10.1016/j.jacc.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997; 337:230–237. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 14.Matetzky S, Freimark D, Ben-Ami S, Goldenberg I, Leor J, Doolman R, et al. Association of elevated homocysteine levels with a higher risk of recurrent coronary events and mortality in patients with acute myocardial infarction. Arch Intern Med 2003; 163:1933–1937. doi: 10.1001/archinte.163.16.1933. [DOI] [PubMed] [Google Scholar]
- 15.Fácila L, Nuñez JE, Vicente Bertomeu G, Sanchis J, Bodi V, Chorro FJ, et al. Early determination of homocysteine levels in acute coronary syndromes, is it an independent prognostic factor? Int J Cardiol 2005; 100:275–279. doi: 10.1016/j.ijcard.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Omland T, Samuelsson A, Hartford M, Herlitz J, Karlsson T, Christensen B, et al. Serum homocysteine concentration as an indicator of survival in patients with acute coronary syndromes. Arch Intern Med 2000; 160:1834–1840. doi: 10.1001/archinte.160.12.1834. [DOI] [PubMed] [Google Scholar]
- 17.Foussas SG, Zairis MN, Makrygiannis SS, Manousakis SJ, Patsourakos NG, Adamopoulou EN, et al. The impact of circulating total homocysteine levels on long-term cardiovascular mortality in patients with acute coronary syndromes. Int J Cardiol 2008; 124:312–318. doi: 10.1016/j.ijcard.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Boysen G, Brander T, Christensen H, Gideon R, Truelsen T. Homocysteine and risk of recurrent stroke. Stroke 2003; 34:1258–1261. doi: 10.1161/01.STR.0000069017.78624.37. [DOI] [PubMed] [Google Scholar]
- 19.Spence JD, Yi Q, Hankey GJ. B vitamins in stroke prevention: time to reconsider. Lancet Neurol 2017; 16:750–760. doi: 10.1016/S1474-4422(17)30180-1. [DOI] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 21.Corti R, Farkouh ME, Badimon JJ. The vulnerable plaque and acute coronary syndromes. Am J Med 2002; 113:668–680. doi: 10.1016/s0002-9343(02)01344-x. [DOI] [PubMed] [Google Scholar]
- 22.Windecker S, Bax JJ, Myat A, Stone GW, Marber MS. Future treatment strategies in ST-segment elevation myocardial infarction. Lancet 2013; 382:644–657. doi: 10.1016/S0140-6736(13)61452-X. [DOI] [PubMed] [Google Scholar]
- 23.Groot HE, Karper JC, Lipsic E, van Veldhuisen DJ, van der Horst ICC, van der Harst P. High-sensitivity C-reactive protein and long term reperfusion success of primary percutaneous intervention in ST-elevation myocardial infarction. Int J Cardiol 2017; 248:51–56. doi: 10.1016/j.ijcard.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Woo KS, Chook P, Lolin YI, Cheung AS, Chan LT, Sun YY, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation 1997; 96:2542–2544. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- 25.Upchurch GR, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 1997; 272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 26.Leng YP, Ma YS, Li XG, Chen RF, Zeng PY, Li XH, et al. l-Homocysteine-induced cathepsin V mediates the vascular endothelial inflammation in hyperhomocysteinaemia. Br J Pharmacol 2018; 175:1157–1172. doi: 10.1111/bph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 1998; 338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 28.Dong M, Zheng N, Ren LJ, Zhou H, Liu J. Increased expression of STIM1/Orai1 in platelets of stroke patients predictive of poor outcomes. Eur J Neurol 2017; 24:912–919. doi: 10.1111/ene.13304. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z, Sarkadi-Nagy E, Bagi Z, Szollar L, Koller A. Simultaneously increased TxA(2) activity in isolated arterioles and platelets of rats with hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 2000; 20:1203–1208. doi: 10.1161/01.atv.20.5.1203. [DOI] [PubMed] [Google Scholar]
- 30.Washio T, Nomoto K, Watanabe I, Tani S, Nagao K, Hirayama A. Relationship between plasma homocysteine levels and congestive heart failure in patients with acute myocardial infarction. Homocysteine and congestive heart failure. Int Heart J 2011; 52:224–228. doi: 10.1536/ihj.52.224. [DOI] [PubMed] [Google Scholar]
- 31.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006; 354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 32.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015; 313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]


