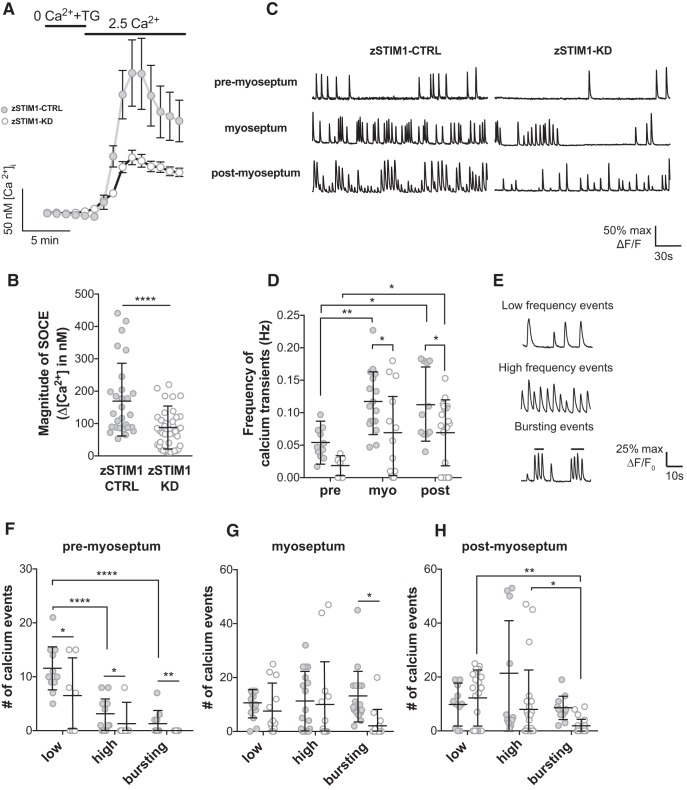
Figure 7.
STIM1 is required for patterning of calcium events in navigating axons in vivo. A, B, fura-2 Ca2+ imaging was used to measure SOCE in spinal motor neurons isolated from zSTIM1 control (zSTIM1-CTRL) and zSTIM1-morphant (zSTIM1-KD) embryos. SOCE was triggered in vitro by incubating neurons in 0 mm Ca2+ media with 5 μm thapsigargin followed by the addition of 2.5 mm Ca2+ media. A, Average fura-2 Ca2+ traces of zSTIM1-CTRL (n = 28) and zSTIM1-KD (n = 42) spinal motor neurons. B, Quantification of SOCE magnitude, calculated as the change in [Ca2+]i upon the addition of 2.5 mm Ca2+ media, in zSTIM1-CTRL and zSTIM1-KD spinal motor neurons (****p = 0.00003, Student's t test). C–H, Axonal calcium events were measured in CaP axons of zSTIM1-CTRL and zSTIM1-KD Gal4s1020t/UAS:GCaMP5G embryos. C, Representative calcium responses from CaP axons of zSTIM1-CTRL or zSTIM1-KD located proximal to the horizontal myoseptum (premyoseptum), at the horizontal myoseptum, or distal to the horizontal myoseptum (postmyoseptum). D, Frequency of calcium events quantified at each stage of axon outgrowth in zSTIM1-CTRL (n = 11 axons from 7 embryos) and zSTIM1-KD embryos (n = 17 axons from 13 embryos). zSTIM1-CTRL axons displayed an increase in frequency of calcium transients at the myoseptum (**p = 0.0035) and postmyoseptum (*p = 0.0104) compared with the premyoseptum. In contrast in zSTIM1-KD axons, the change in frequency of calcium transients only increased at the postmyoseptum compared with the premyoseptum (*p = 0.0403). Significantly, reduced STIM1 expression attenuated the frequency of calcium events at the myoseptum (*p = 0.0298) and postmyoseptum (*p = 0. 0.0453), compared with zSTIM1-CTRL (two-way ANOVA with Holm–Sidak test for multiple comparisons). E, Representative traces illustrate low-frequency (<7.5 spikes·min−1), high-frequency (≥7.5 spikes·min−1), and bursting (≥15 spikes·min−1) calcium events observed in CaP axons. F–H, Calcium events were quantified in CaP axons of zSTIM1-CTRL and zSTIM1-KD. F, At the premyoseptum, low-frequency calcium activity predominated and was significantly reduced in zSTIM1-KD compared with zSTIM1-CTRL (*p = 0.0238). G, At the myoseptum, bursting calcium activity was significantly reduced in zSTIM1-KD compared with zSTIM1-CTRL (*p = 0.0212). H, At the postmyoseptum, bursting calcium activity was significantly reduced in zSTIM1-KD compared with zSTIM1-CTRL (*p = 0.0477, two-way ANOVA with Holm–Sidak test for multiple comparisons). A, The key applies to graphs in B, C, F–H: closed circles represent zSTIM1-CTRL; open circles represent zSTIM1-KD.