Figure 5.
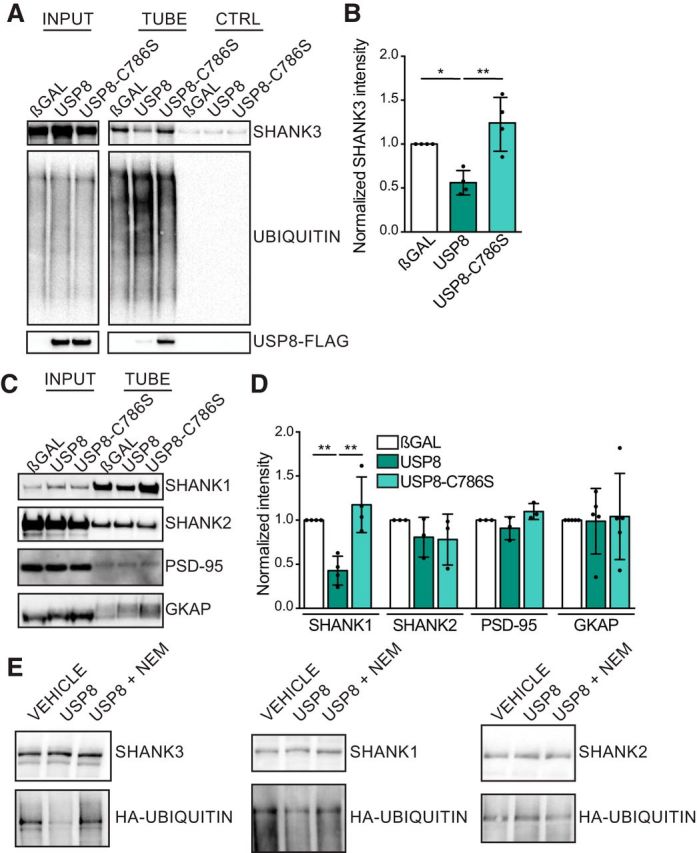
USP8 regulates the ubiquitination of SHANK3 and SHANK1. A, Representative immunoblots from assays that used TUBEs coupled to magnetic beads to pull down polyubiquitinated proteins. Stable SHANK3-expressing cells were transfected with βGAL control, USP8, or USP8–C786S (enzymatic dead) and were treated with the proteasome inhibitor MG132 for 3 h before lysis and pulldown. B, Intensity of SHANK3 immunoblot bands from pulldown lanes were quantified. Overexpression of USP8 leads to a decrease in the level of polyubiquitinated SHANK3 relative to βGAL control or USP8–C786S. n = 4 assays from independent cultures. One-way ANOVA: F = 12.63, p = 0.0024; βGAL vs USP8, p = 0.0264; βGAL vs C786S, p = 0.2388; USP8 vs C786S, p = 0.0020. C, Representative immunoblots of TUBE pulldown assays from HEK293 lysates transiently transfected with SHANK1, SHANK2, PSD-95, or GKAP along with βGAL, USP8, or USP8–C786S. D, Quantification of TUBE pulldown assays from C. SHANK1 was less ubiquitinated when USP8 was overexpressed relative to βGAL control or USP8–C786S. SHANK2, PSD-95, and GKAP polyubiquitination were unaffected by USP8 overexpression. n = 3–5 assays from independent cultures. SHANK1: one-way ANOVA: F = 14.53, p = 0.0015; βGAL vs USP8, p = 0.0085; βGAL vs C786S, p = 0.4814; USP8 vs C786S, p = 0.0016; SHANK2: one-way ANOVA: F = 0.9666, p = 0.4326; PSD-95: one-way ANOVA: F = 3.235, p = 0.5189; GKAP: one-way ANOVA: F = 0.03123, p = 0.9693. E, Ubiquitinated SHANK proteins were immunoprecipitated from HEK293 cells overexpressing SHANK1, SHANK2, or SHANK3 together with HA-ubiquitin and treated with MG132. Immunoprecipitated SHANK1, SHANK2, or SHANK3 was incubated with vehicle, recombinant USP8, or recombinant USP8 plus the DUB inhibitor NEM in an in vitro deubiquitination assay. HA-ubiquitin signal on SHANK3 and SHANK1 was reduced by recombinant USP8. *p < 0.05, **p < 0.01.
