Figure 4.
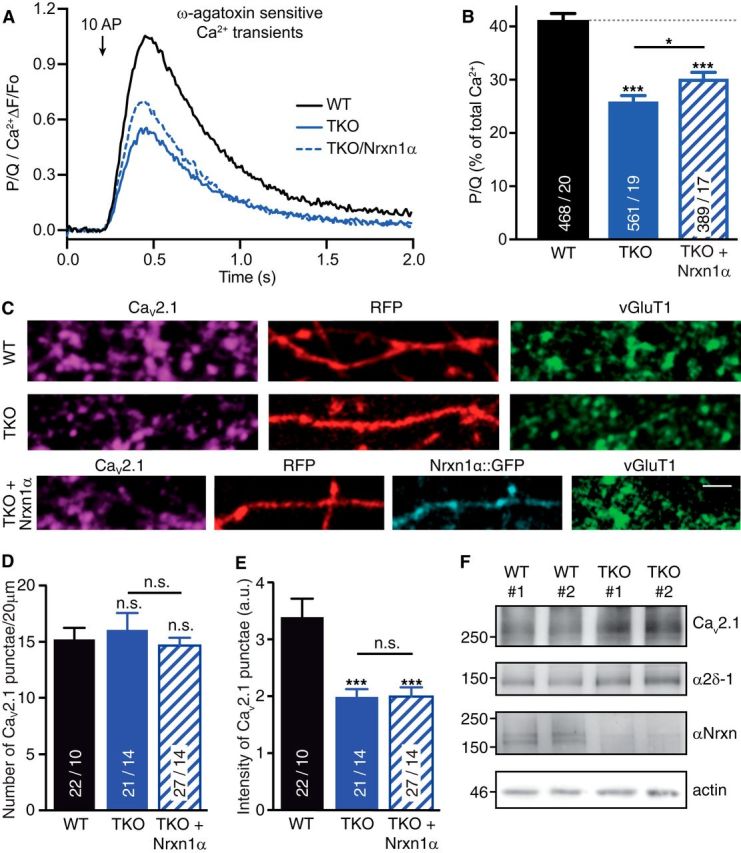
Deletion of αNrxns leads to reduced CaV2.1-mediated presynaptic Ca2+ influx and channel abundance. A, Isolated ω-agatoxin IVA-sensitive traces of Ca2+ transients through CaV2.1 (P/Q-type) channels derived from subtraction of transients before and after addition of the CaV2.1 blocker. Traces are recorded by synGCaMP6f and averaged across multiple boutons of WT, TKO, and TKO neurons transfected with Nrxn1α (TKO+Nrxn1α). B, Summary histogram of CaV2.1 channel contributions (in percentage of total Ca2+ transients) of WT boutons compared with TKO, and TKO+Nrxn1α. Data are mean ± SEM; n = ROIs/neurons (in bars), differences to WT (dotted line) are indicated above columns; ***p < 0.001; *p = 0.042, by one-way ANOVA, F(2,1415) = 42.6. C, Representative images of immunofluorescence with antibodies against endogenous α1A of CaV2.1 (magenta), colabeled against vesicular glutamate transporter (vGluT1, green) in WT and TKO neurons transfected with RFP (red) alone (WT, TKO) or in combination with Nrxn1α::GFP (cyan, TKO+Nrxn1α). Scale bar, 2.5 μm. D, Quantification of the number of α1A-positive puncta that colocalize with presynaptic vGluT1 along RFP-filled axons of WT, TKO and TKO+Nrxn1α neurons. E, Quantification of immunofluorescence intensity of α1A-positive puncta colocalizing with vGluT1. Data in D and E are mean ± SEM; n = number of axonal segments (shown in bars) from at least three independent experiments. **p < 0.01; ***p < 0.001, n.s. = not significant, by one-way ANOVA. F, Immunoblots of total protein lysates from two independent WT and TKO cultures, each representing ∼300,000 hippocampal cells. Blots were probed with antibodies against the α1A pore forming and α2δ-1 auxiliary subunits of CaV2.1, and against all αNrxn variants that are deleted in TKO; actin = loading control.
