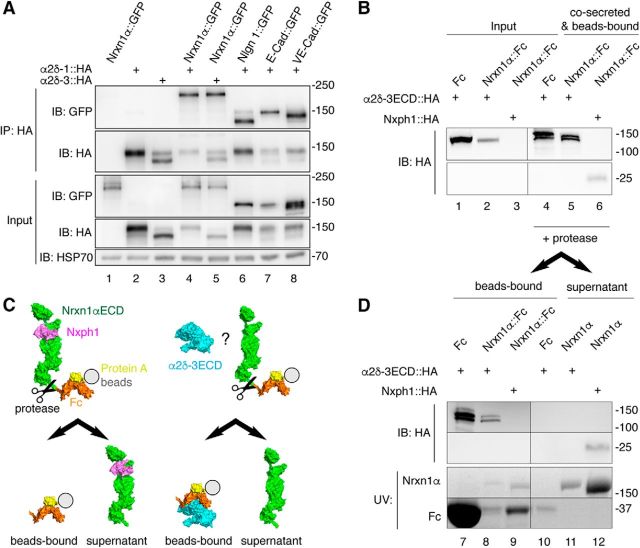
Figure 9.
Nrxn1α does not engage in stable complexes with α2δ subunits. A, IP of cotransfected α2δ subunits and Nrxn1α or control membrane proteins from HEK293 cell lysates (top). IPs of HA-tagged α2δ-1 and α2δ-3 enrich Nrxn1α::GFP (lanes 4, 5) similar to the controls neuroligin-1 (Nlgn1), E-cadherin (E-Cad) and VE-cadherin (VE-Cad; lanes 6–8). Single transfections served as control for antibody specificity (lanes 1–3). Endogenous HSP70 indicates equal amounts of lysates used (botto). B, Co-secretion of extracellular domains of α2δ-3 (α2δ-3ECD::HA) and Nrxn1α (Nrxn1α::Fc) into HEK293 cell medium with subsequent binding of the Fc moiety to protein A beads. Lysates of cells show α2δ-3ECD::HA (lanes 1–2). Whereas the positive control, Nxph1-HA, is hardly detectable in cell lysates (lane 3, bottom), it is enriched with Nrxn1α::Fc (lane 6, bottom). α2δ-3ECD::HA is enriched similarly with Nrxn1α::Fc (lane 5, top) but also with the Fc-tag alone. C, Diagram of the cleavage experiment using HRV 3C protease to release the Nrxn1αECD from Fc-beads (immunoblot data in D). Left, Nxph1 (magenta) is bound to Nrxn1αECD (green) as expected. Right, α2δ-3ECD (cyan) remains on Fc-coupled beads (orange) but does not interact with Nrxn1αECD. D, Immunoblot of the cleavage experiment (C) that starts from the precipitated samples in B. After addition of protease, α2δ-3ECD remains on Fc-tag bound to beads (lanes 7, 8) but is not found on Nrxn1αECD in the supernatant (lane 11). The positive control, Nxph1, is bound to the released Nrxn1αECD (lane 12). α2δ-3 and Nph1 are shown by immunoblot, Nrxn1α and Fc proteins are visualized by UV light.