Figure 2.
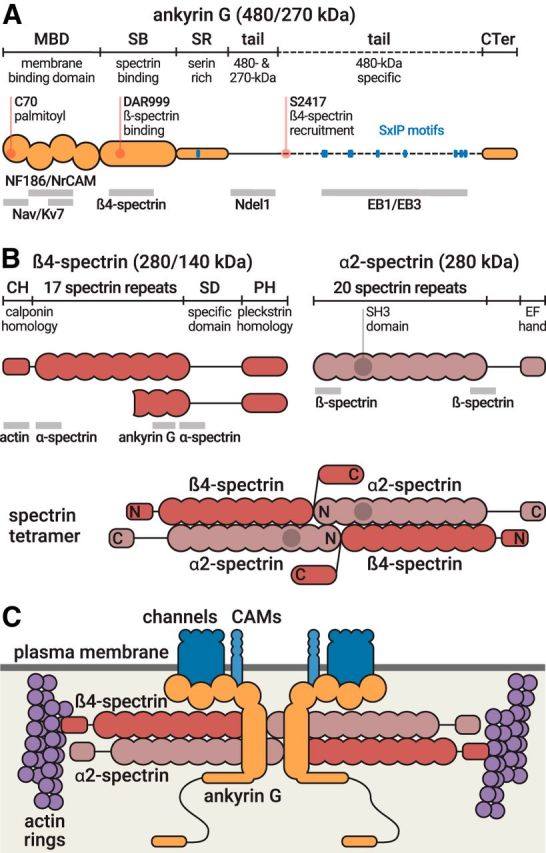
Molecular structure of ankyrin G and AIS spectrins. A, Domain organization of ankyrin G, which exists as isoforms of 480 and 270 kDa at the AIS (top, orange). Important residues (red) and EB-binding SxIP motifs (blue) are indicated. Binding sites of partners are indicated below the protein (gray bars). B, Domain organization of β4-spectrin, which exists as isoforms of 280 and 140 kDa (left), and domain organization of α2-spectrin (right). Binding sites of partners are indicated below the protein (gray bars). Bottom, Structure of the α2/β4 spectrin tetramer. N and C indicate the aminoterminus and carboxyterminus, respectively, of each subunit. C, The AIS submembrane complex. The α2/β4 spectrin tetramers (red) lie horizontally under the plasma membrane (dark gray), connecting actin rings (purple) with a distance of ∼190 nm. In the middle of the tetramer, ankyrin G (orange) is bound to β4-spectrin and anchors AIS membrane proteins (Nav/Kv7 channels, CAMs, blue).
