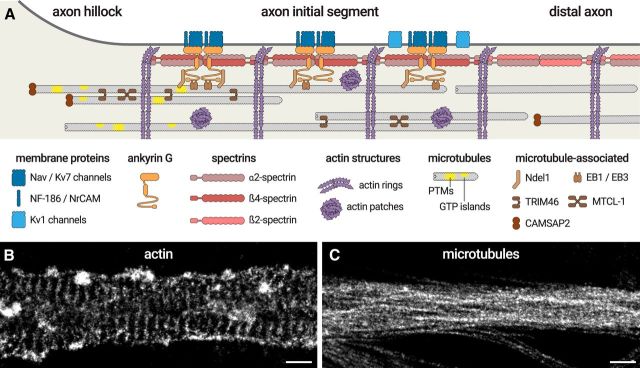
Figure 3.
The molecular organization of the AIS. A, Diagram depicting the organization of the AIS scaffold. Membrane proteins (Nav/Kv7 channels, NF-186, NrCAM, blue) are anchored by ankyrin G (orange), itself inserted into the α2/β4-spectrin complex (red) that spaces actin rings (purple). Kv1 channels (light blue) are present along the distal AIS. In the distal axon, the complex is made of α2- and β2-spectrin (pink). Ankyrin G binds to microtubules via EB1/EB3 proteins and Ndel1 (brown). AIS microtubules (gray) are capped by CAMSAP2 at their minus-end (brown) and grouped in fascicles crosslinked by TRIM46 and possibly MTCL-1 (brown). They are enriched in post-translational modifications (PTMs) and GTP islands (yellow). Intracellular patches of actin (purple) are also present inside the AIS. B, The <50 nm resolution of stochastic optical reconstruction microscopy (STORM) makes it possible to resolve the 190 nm spacing of actin rings and the presence of actin patches along the AIS. C, STORM can resolve microtubule bundles along the AIS, but the close apposition within bundles (∼30 nm spacing) makes it a challenge to distinguish individual microtubules. Scale bars: B, C, 0.5 μm.