FIGURE 3. Vascular Normalization Can Reprogram the Immunosuppressive Tumor Microenvironment.
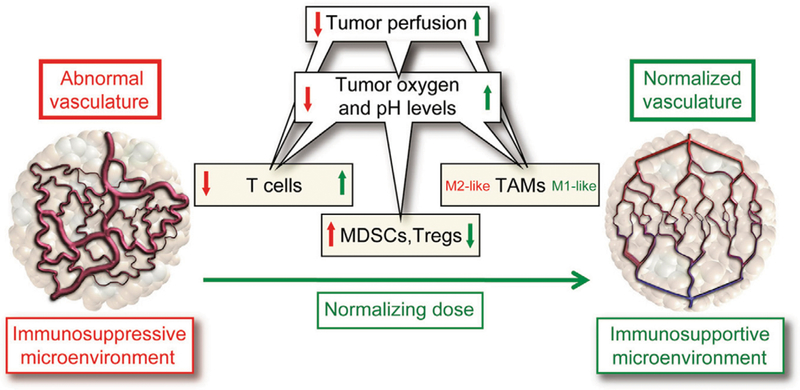
The abnormal tumor vasculature, induced by overexpression of VEGF, can inhibit effector T cells, preferentially recruit immunosuppressive immune cells (e.g., myeloid-derived suppressor cells [MDSCs], regulatory T cells [Tregs], and “M2-like” protumor tumor-associated macrophages [TAMs]), and promote hypoxia, thus establishing an immunosuppressive tumor microenvironment. Judicious dosing of antiangiogenic therapies (such as anti-VEGF antibodies) can reprogram the tumor microenvironment to an immunostimulatory milieu by normalizing the vasculature to facilitate T effector cell infiltration and antitumor function, reduce MDSC and Treg accumulation, and alleviate hypoxia, which can induce conversion of TAMs to an “M1-like” antitumor phenotype. Reprinted with permission from Jain.3
