Figure 1.
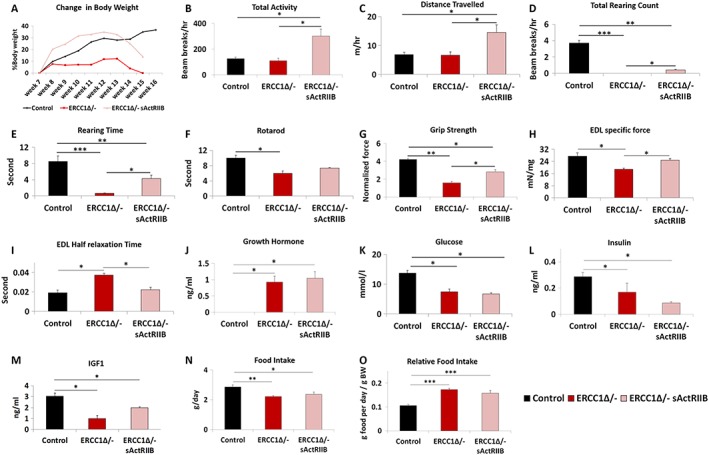
sActRIIB treatment mitigates body, whole animal activity, grip strength, losses, and specific force loss in Ercc1 Δ/− mice. (A) Relative changes in body mass over time. Intraperitoneal injection of Ercc1 Δ/− with sActRIIB started at week 7 and tissues collected at the end of week 15. Organismal activity measurements through activity cages. Measurements in (B–E) made at the end of week 14. (F) Rotarod activity. (G) Muscle contraction measurement through assessment of grip strength. (H) Ex vivo assessment of EDL‐specific force. (I) Half relaxation time for the EDL. Levels of (J) growth hormone, (K) glucose, (L) insulin, and (M) insulin‐like growth factor‐1 at beginning of week 15. (N) Food intake and (O) relative food intake at the end of week 15. n = 6 control male mice, n = 5 Ercc1 Δ/− untreated male mice, and n = 5 Ercc1 Δ/− treated male mice. All analysis performed using non‐parametric Kruskal–Wallis test followed by the Dunn's multiple comparisons except (J) where one‐way analysis of variance followed by Bonferroni's multiple comparison tests was used. *P < 0.05, **P < 0.01, ***P < 0.001. EDL, extensor digitorum longus; IGF‐1, insulin‐like growth factor‐1; sActRIIB, soluble activin receptor type IIB.
