Figure 7.
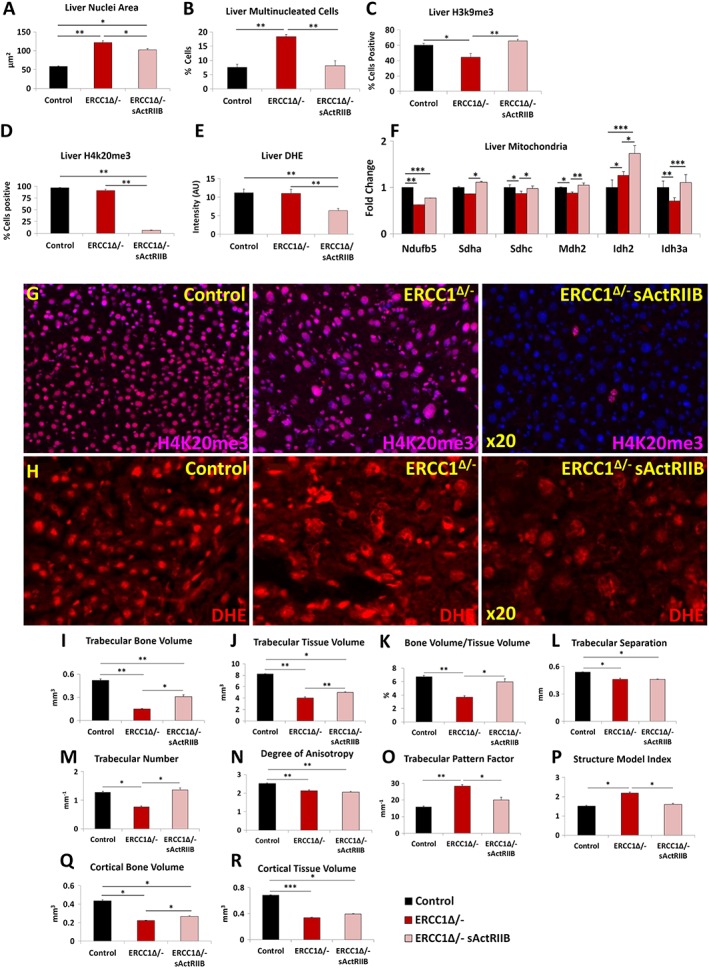
sActRIIB prevents the development age‐related liver abnormalities and osteoporotic phenotype in Ercc1 Δ/−. (A) Measure of liver nuclear size. (B) Profile frequency of multinucleated liver cells. (C) Frequency of H3K9me3‐positive liver cells. (D) Frequency of H4K20me3‐positive liver cells. (E) Quantification of DHE fluorescence to gauge superoxide levels. (F) Quantitative PCR profiling of mitochondrial gene expression. (G) Immunofluorescence images for H4K20me3 distribution in the three cohorts. (H) DHE intensity levels in the three cohorts. (I) Trabecular bone volume measurements. (J) Trabecular tissue volume measurements. (K) Trabecular bone to tissue volume ratios. (L) Trabecular separation indices. (M) Enumeration of trabeculae. (N) Degrees of trabecular anisotrophy. (O) Trabecular pattern factor as a quantification of bone architecture. (P) Structure model index. (Q) Measure of cortical bone volume. (R) Cortical tissue volume measure. Trabecular bone volume measurements. n = 8 for all animals in (A–H) and n = 6 control male mice, five Ercc1 Δ/−‐untreated male mice, and six Ercc1 Δ/−‐treated male mice in other experiments. One‐way analysis of variance followed by Bonferroni's multiple comparison tests used for (A–F) and non‐parametric Kruskal–Wallis test followed by the Dunn's multiple comparisons for (I–R). *P < 0.05, **P < 0.01, ***P < 0.001. DHE, dihydroethidium; sActRIIB, soluble activin receptor type IIB.
