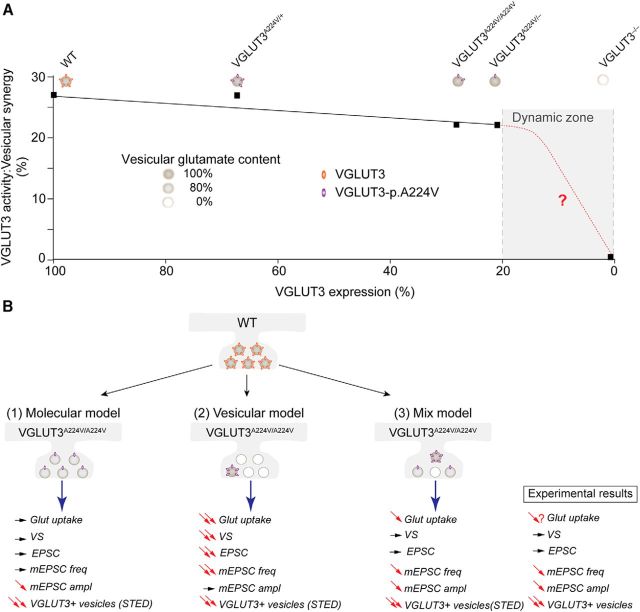
Figure 10.
Putative models depicting the reduction in VGLUT3 at the synapses of different mouse genotypes. In this study, we investigated mouse models with variable levels of VGLUT3 expression: WT mice (2 copies of WT VGLUT3 isoform; 100%), heterozygous mice (1 copy of WT VGLUT3 isoform and 1 copy of VGLUT3-p.A224V allele; 67%), homozygous mice (2 copies of the mutated VGLUT3-p.A224V allele; 28%), VGLUT3A224V/− mice (expressing only 1 copy of the mutated VGLUT3-p.A224V allele; 21%), and VGLUT3 knock-out mice (no copy of VGLUT3; 0%). A, The black curve shows the relationship between VGLUT3 expression (detected by immunoautoradiography) and the activity of VGLUT3 (indirectly assessed by measuring vesicular synergy). VGLUT3 activity did not decline in proportion to the amount of VGLUT3 in the expression range between 100% and 21% (black curve). The absence of a correlation between these two sets of measurements explains the virtual lack of a VGLUT3-dependent phenotype in our panel of mutants. This model predicts that these phenotypes will be observed in the gray zone of the curve (red curve). B, Three putative models that may account for the reduction in VGLUT3 at the synapses are compared with our experimental results. In WT mice, numerous copies of VGLUT3 are uniformly distributed between synaptic vesicles, and these vesicles are “normally” loaded (100% gray level) with glutamate. In Model 1 (molecular model), VGLUT3 copies are uniformly decreased in all vesicles of VGLUT3A224V/A224V mice. In this model, the vesicular content of glutamate is minimally decreased in all vesicles. This decrease in glutamatergic quantal size cannot be observed with bulk methods, such as vesicular uptake or behavioral measurements, but can be detected by more sensitive electrophysiological techniques. Model 1 would be compatible with (1) an absence of change in vesicular uptake (observed in BON cells) and vesicular synergy (VS; observed in cortical vesicles) and (2) a decrease in the amplitudes of the mEPSCs that were observed in recordings of isolated neurons. Model 2 (vesicular model) is based on the STED high-resolution inspection of VGLUT3-positive terminals in our panel of mutants. In this model, in a small proportion of vesicles, the number of VGLUT3-p.A224V copies per vesicles is similar (or minimally decreased) to that found in WT neurons. According to the electrophysiological recordings of autapses, these vesicles may contain normal levels of glutamate and be preferentially docked. The remaining vesicles (80%) will contain neither VGLUT3 nor glutamate. This model is consistent with the decreased frequency of the mEPSCs. A mixed model (Model 3) of these two models may better explain all of our experimental results. In this model, we found vesicles without VGLUT3, a small proportion of vesicles expressing the correct number of VGLUT3-p.A224V copies, and a third population of vesicles in which the number of VGLUT3 copies were uniformly decreased in all vesicles of the synapses from VGLUT3A224V/A224V mice.