Figure 6.
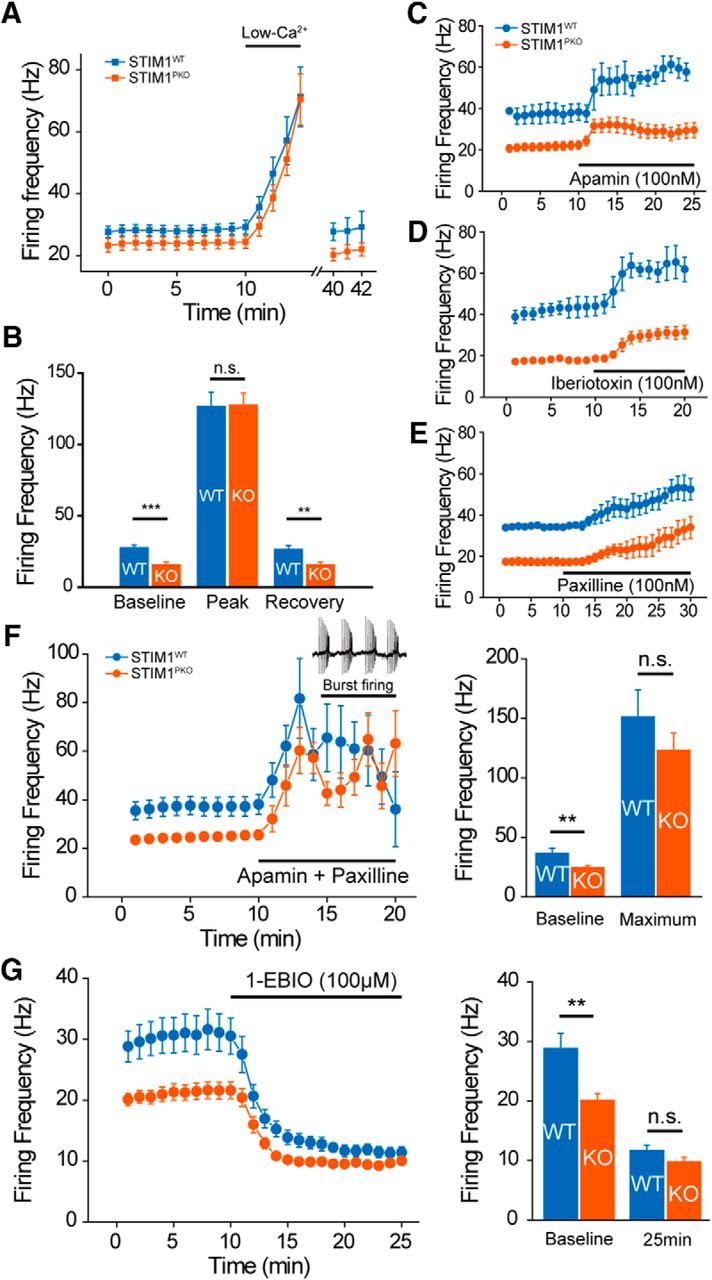
Altered excitability of STIM1PKO PNs required activation of Ca2+-activated K+ channels. A, Continuous cell-attached recording during the application of ACSF containing low Ca2+ concentration. Under low extracellular Ca2+ concentration (100 μm) firing rates of both groups increased and reached a similar peak point within 5 min. When extracellular Ca2+ concentration returned to normal (2 mm), the firing rate slowly recovered to the initial level (wild-type, n = 15; STIM1PKO, n = 14). B, Bar graph of low-Ca2+ contained ACSF application (right). Though PNs of STIM1PKO mice had significantly lower baseline firing rates than wild-type littermates at baseline (p < 0.001), the firing rates finally met at the peak (p = 0.949). When the firing rate recovered under normal ACSF, the gap between wild-type and STIM1PKO mice was also recovered (p = 0.003). C, Blocking SK channel by apamin (100 nm) increases the firing rates of both groups of PNs (wild-type, n = 3; STIM1PKO, n = 13). D, E, Blocking BK channel also raised spontaneous firing rate in both groups. D, Application of iberiotoxin (100 nm; wild-type, n = 3; STIM1PKO, n = 4). E, Paxilline, a broader spectrum blocker that is able to block iberiotoxin-insensitive BK currents (100 nm; wild-type, n = 2; STIM1PKO, n = 6) were not able to reduce the firing rate difference between two groups. F, Blocking both SK and BK channels with the cocktail of apamin (100 nm) and paxilline (100 nm). The mixture of drugs narrows the gap in firing rates between wild-type and STIM1PKO mice (wild-type, n = 7; STIM1PKO, n = 9). Although blocking both SK and BK channels suddenly changes the firing pattern (from tonic firing to burst firing), the firing frequencies of both groups became similar before pattern change (left). Bar graph in two time points (right). Baseline is before drug treatment, and maximum is the maximum firing rate before pattern change. In the comparison of maximum firing rates, there was no statistical significance between wild-type and STIM1PKO (baseline, p = 0.003; maximum, p = 0.351). G, Potentiating KCa channels with 1-EBIO (100 μm). 1-EBIO treatment decreased the difference between wild-type and STIM1PKO mice without changing the firing pattern (left; wild-type, n = 7; STIM1PKO, n = 15). The bar graph show the baseline and 15 min after drug application (right). At 15 min after 1-EBIO treatment, statistical significance between wild-type and STIM1PKO mice disappeared (baseline, p = 0.004; 25 min, p = 0.078). Asterisks in all bar graphs were marked by the Mann–Whitney U test. Error bars denote the SEM. **p < 0.01; ***p < 0.001.
