Figure 2.
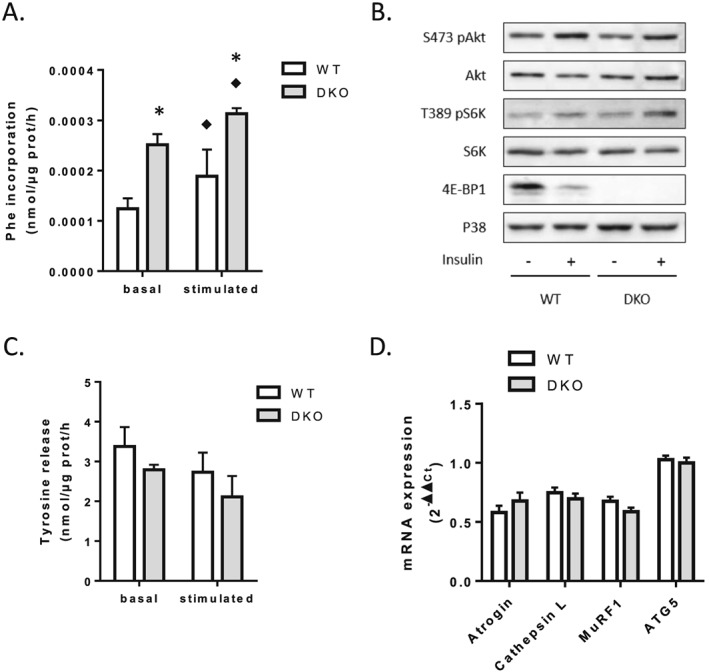
Loss of 4E‐BP1 and 4E‐BP2 alters protein homeostasis in skeletal muscle. (A) Protein synthesis in WT and 4E‐BP1/2 DKO skeletal muscles. Protein synthesis was measured ex vivo by radioactive phenylalanine incorporation in extensor digitorum longus with (stimulated) or without (basal) stimulation with a mixture of leucine and insulin. (B) Representative western blot levels of Akt, S6K, and 4E‐BP1 phosphorylation in WT and 4E‐BP1/2 DKO quadriceps. Mice were fasted overnight before receiving a 1.2 mU/g body weight intraperitoneal insulin injection. Mice were sacrificed 20 min later, and tissues were collected for western blot analysis. (C) Proteolysis in WT and 4E‐BP1/2 DKO skeletal muscles. Proteolysis was analysed ex vivo in extensor digitorum longus by measuring tyrosine release as described in the materials and methods Materials and methods. (D) Real‐time PCR quantification of Atrogin/MAFbx, Cathepsin L, MuRF1, and ATG5 mRNA expression in quadriceps from WT and 4E‐BP1/2 DKO mice. n = 5–6 in each genotype, p values were assessed by two‐way analysis of variance, and Bonferroni post‐tests were used to compare replicate means by row (in A–C) or by unpaired t‐test (in D). ♦P < 0.05 vs. basal, * P < 0.05 vs. WT.
