Figure 3. B27-and gut-associated mechanisms that may contribute to AAU pathogenesis.
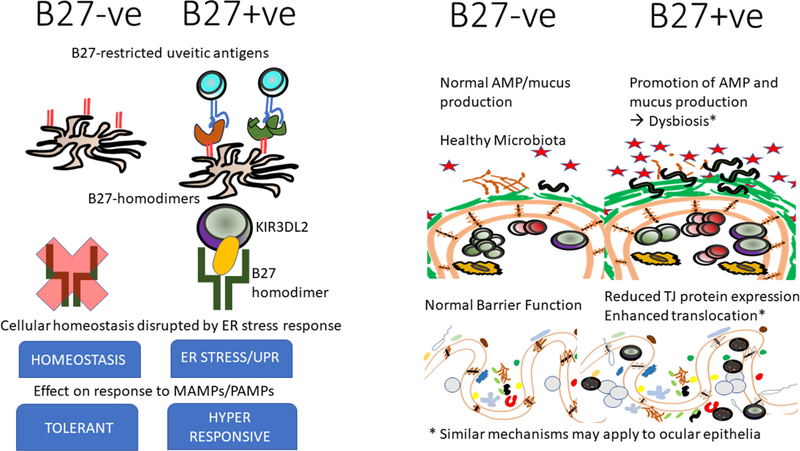
a) HLA B27-associated mechanisms. Figure shows HLA B27-associated changes that may accompany HLA B27 expression either at the cellular (left side) or tissue (right side) level that may predispose HLA-B27 +ve individuals to a loss of gut and/or ocular tolerance.
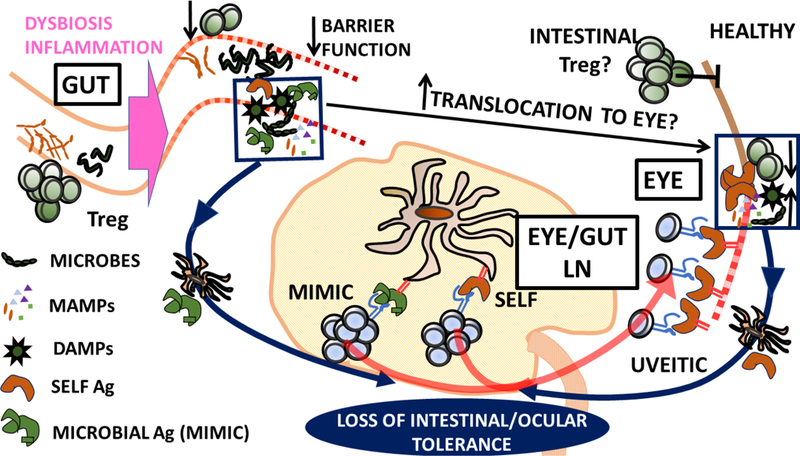
b) Self-Ag dependent model of uveitis. Dysbiosis leads to gut inflammation. Reduction of intestinal Treg that may normally contribute to ocular homeostasis or dissemination of intestinal microbes/MAMPs/DAMPs (arising from tissue damage and increased permeability) that act as adjuvants in the eye may result. Loss of ocular immune privilege leads to activation of uveitic self-reactive T cells in ocular draining LN. Loss of gut tolerance may also drive activation of T cells reactive to microbial antigens with mimicry to self in gut draining LN. These T cells subsequently target self-Ag in the eye.
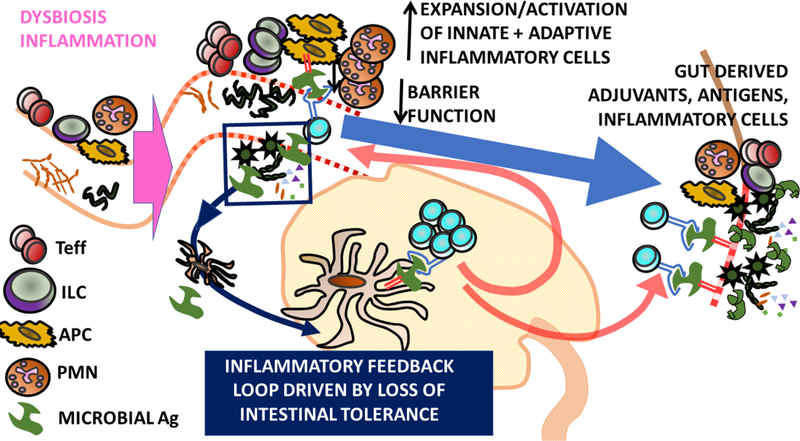
c) Self-Ag independent model of uveitis. Dysbiosis and/or intestinal inflammation leads to the expansion of intestinal effector immune cells, e.g. conventional and non-conventional effector T cells (Teff), polymorphonuclear cells (PMN), innate lymphoid cells (ILC) and antigen presenting cells (APC). This developing inflammatory environment may be exacerbated by the loss of intestinal tolerance and expansion of microbially-reactive T cells which drive an inflammatory feedback loop. Diminished barrier function may promote dissemination of microbial products from gut to eye. Activation/Expansion of inflammatory immune cells in the intestine may promote their recirculation and potential migration to the eye. Antigen presentation of microbial antigens in the eye may further drive inflammation.
Note by no means are the two models proposed mutually exclusive.
Abbreviations: Ag, antigen; DAMP, damage-associated molecular pattern; LN; lymph node; MAMP, microbe-associated molecular pattern; Treg. regulatory T cell.
