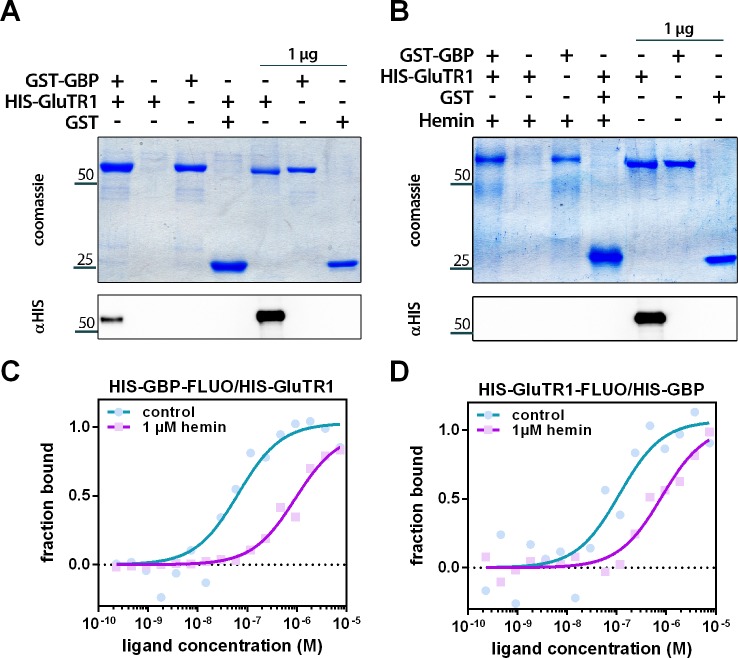
Figure 7. Hemin interferes with the binding of GBP to GluTR1.
(A) Pulldown of 6xHIS-GluTR1 by GST-GBP. Both proteins were incubated in the presence of Glutathione-Sepharose (GS), washed, eluted using reduced glutathione and separated on a 12% SDS-polyacrylamide gel. Eluates of reactions containing GST-GBP alone or GST and GluTR1 as well as 1 µg of the input (no GS incubation) were loaded as controls. Note that 6xHIS-GluTR1 and GST-GBP show almost identical migration behavior. Successful pulldown of 6xHIS-GluTR1 by GST-GBP was confirmed using a HIS-tag-specific antibody (αHIS) after western blotting. (B) Same experiment as in (A) but carried out in the presence of 500 µM hemin. Note that hemin prevents pulldown of 6xHIS-GluTR1 by GST-BP. (C) Microscale thermophoresis (MST) of the fluorophore-labeled (FLUO) 6xHIS-GBP titrated against increasing amounts of 6xHIS-GluTR1. The affinity of 6xHIS-GBP-FLUO for 6xHIS-GluTR1 (Kd = 65 nM) decreases upon addition of 1 µM hemin (Kd = 931 nM). n = 2 independent experiments. (D) MST of fluorophore-labeled 6xHIS-GluTR1-FLUO titrated against increasing amounts of 6xHIS-GBP. The affinity of 6xHIS-GluTR1-FLUO for 6xHIS-GBP (Kd = 113 nM) decreases upon addition of 1 µM hemin (Kd = 806 nM). n = 2 independent experiments.
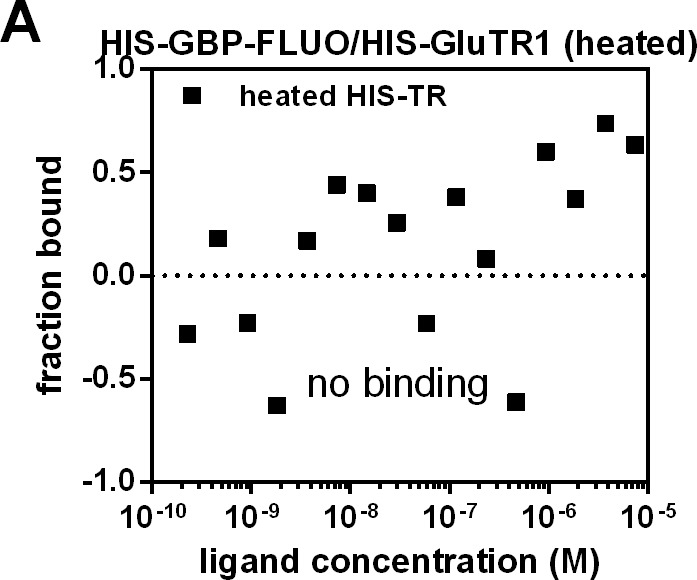
Figure 7—figure supplement 1. Control for MST measurement.