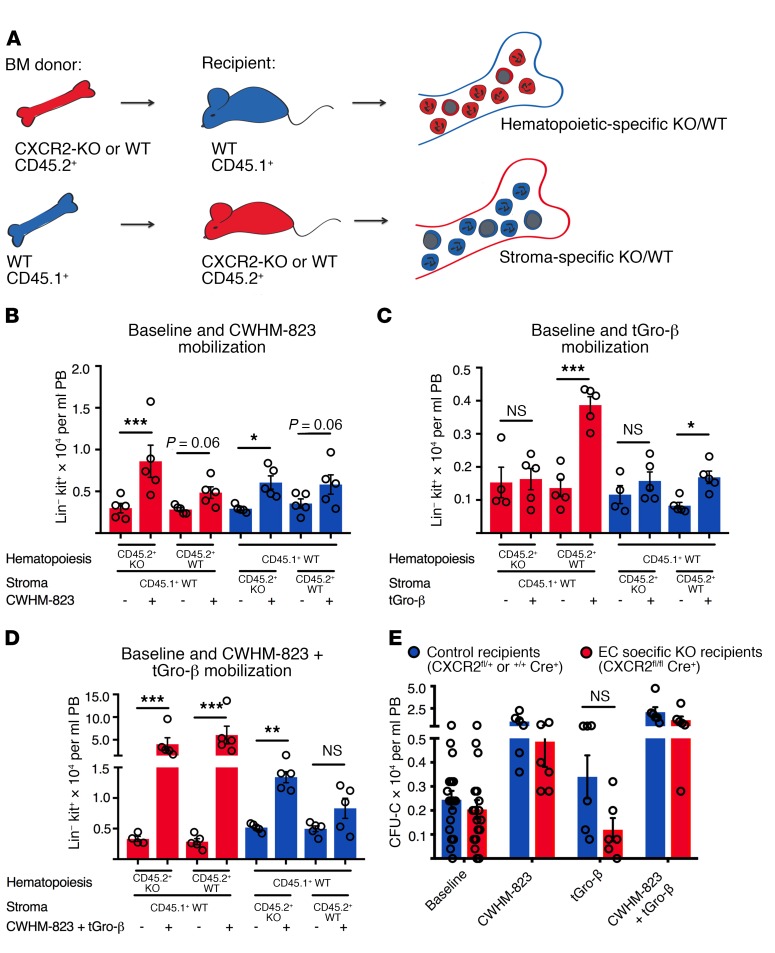
Figure 4. CXCR2 expression in both the hematopoietic and nonhematopoietic (stromal) compartment contributes to tGro-induced mobilization.
(A) Hematopoietic-specific CXCR2-KO mice were generated by transplanting lethally irradiated CD45.1+ recipients (BALB/cJ) with CD45.2+ BM cells isolated from CXCR2-KO mice (BALB/c, 1 × 106 cells per recipient, n = 3 BM donor mice). A control group was reconstituted with CD45.2+ WT BM cells (BALB/c, 1 × 106 cells per recipient, n = 3 BM donor mice). Stromal-specific knockouts were generated by transplanting CD45.1+ WT BM cells (BALB/cJ, 1 × 106 cells per recipient, n = 3 BM donors) into CXCR2-KO mice (CD45.2+). In the corresponding control group, WT CD45.2+ recipients were transplanted with the WT CD45.1+ BM graft. (B–D) Three months after transplantation, circulating HSPC numbers (Lin-kit+ cells) were assessed in the different recipients at baseline and following mobilization with CWHM-823 alone (3 mg/kg, 1 hour after s.c. injection, B), tGro-β alone (2.5 mg/kg, 15 minutes after s.c. injection, C), and both agents combined (dosed as indicated for separate treatments, 30 minutes after simultaneous s.c. injection, D). Each bar is mean ± SEM, n = 4–5. ***P < 0.001, **P < 0.01, *P < 0.05. (E) Lethally irradiated CXCR2fl/flCdh5Cre+ hosts (C57BL/6 background, CD45.2+) were reconstituted with syngeneic WT CD45.1+ BM (3 × 106 cells per recipient) to generate EC-specific knockout recipients. In the control group, CXCR2fl/+Cre+ and CXCR2+/+ Cre+ mice were used as recipients. Three months after transplantation, circulating HSPC (CFU-C) numbers were quantified at baseline and following mobilization with CWHM-823 alone (3 mg/kg, 1 hour after s.c. injection), tGro-β alone (2.5 mg/kg, 15 minutes after s.c. injection), and both agents combined (dosed as indicated for separate treatment, 30 minutes after simultaneous s.c. injection). Each bar is mean ± SEM, n = 6. Statistical comparisons were made using ANOVA, followed by step-down Bonferroni’s adjustment for multiple comparisons. Logarithm transformation was performed for the data in D and E.