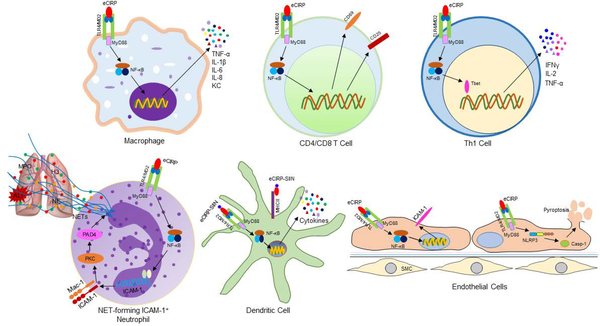
Figure 2: Proinflammatory effects of eCIRP.
eCIRP binds to its receptor TLR4/MD2 complex expressed in various cell types. In macrophages and lymphocytes, eCIRP activates NF-κB through TLR4/MD2 complex and increases the expression of proinflammatory cytokines and chemokines, and T cell activation markers such as CD69 and CD25. eCIRP skews proinflammatory Th1 type T cells by activating the master transcription factor T-bet. In neutrophils, through the classical TLR4/MD2 and NF-κB pathways eCIRP upregulates the expression of ICAM-1 on their surface. ICAM-1 transduces downstream signaling to activate PAD4-dependent NET formation. Increased NETosis by ICAM-1+ neutrophils in turn causes acute lung injury. In the DCs, a CIRP fusion protein prepared by combining amino acids of CD8 T cell’s TCR specific epitope from OVA and three amino-acid flanking residues linked to the N-terminus of murine CIRP named SIIN-CIRP induces DC maturation, cytokine production and migration in a TLR4-dependent manner. SIIN-CIRP protein also enhances antigen presentation. In the ECs, eCIRP increases the expression of ICAM-1 through TLR4/MD2 and NF-κB pathways. In addition, eCIRP induces NLRP3 inflammasome through TLR4/MD2 and NF-κB-dependent pathways. Increased activation of inflammasome leads to caspase-1 activation and subsequently IL-1β and IL-18 production and pyroptosis. eCIRP, extracellular cold-inducible RNA-binding protein; TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; MyD88, myeloid differentiation factor 88; ICAM-1, intercellular cell adhesion molecule-1; DCs, dendritic cells; ECs, endothelial cells; SMC, smooth muscle cells; PAD4, peptidyl arginase deaminase 4; NLRP3, NACHT, LRR and PYD domains-containing protein 3.