Abstract
Pelvic organ prolapse is characterized as the descent of the pelvic organs into the vaginal canal. In the USA, there is a 12% lifetime risk for requiring surgical intervention. Although vaginal childbirth is a well-established risk factor for prolapse, the underlying mechanisms are not fully understood. Decreased smooth muscle organization, composition and maximum muscle tone are characteristics of prolapsed vaginal tissue. Maximum muscle tone of the vaginal wall was previously investigated in the circumferential or axial direction under uniaxial loading; however, the vaginal wall is subjected to multiaxial loads. Further, the contribution of vaginal smooth muscle basal (resting) tone to mechanical function remains undetermined. The objectives of this study were to determine the contribution of smooth muscle basal and maximum tone to the regional biaxial mechanical behaviour of the murine vagina. Vaginal tissue from C57BL/6 mice was subjected to extension–inflation protocols (n = 10) with and without basal smooth muscle tone. Maximum tone was induced with KCl under various circumferential (n = 5) and axial (n = 5) loading conditions. The microstructure was visualized with multiphoton microscopy (n = 1), multiaxial histology (n = 4) and multiaxial immunohistochemistry (n = 4). Smooth muscle basal tone decreased material stiffness and increased anisotropy. In addition, maximum vaginal tone was decreased with increasing intraluminal pressures. This study demonstrated that vaginal muscle tone contributed to the biaxial mechanical response of murine vaginal tissue. This may be important in further elucidating the underlying mechanisms of prolapse, in order to improve current preventative and treatment strategies.
Keywords: vagina, contractility, regional, smooth muscle, basal tone, biaxial
1. Introduction
The female pelvic organs (uterus, bladder and rectum) are supported by pelvic floor muscles and fibromuscular tissues. Weakening of these structures may lead to the descent of the pelvic organs into the vaginal canal, known as pelvic organ prolapse (POP). In the USA, there is a 12% lifetime risk for women to undergo pelvic reconstructive surgery to restore the anatomical position of the pelvic organs to alleviate symptoms (discomfort, defecatory dysfunction, incomplete bladder emptying, etc.) [1]. Clinical intervention for POP results in an annual expenditure of over US$1 billion [2]. Although vaginal childbirth, ageing and increased abdominal loading are well-established risk factors [3,4], the underlying mechanisms for POP are not fully understood.
The levator ani, a group of striated muscles, provides constant tone and structural support to the pelvic organs [5]. Injury during childbirth resulting in reduced muscle tone is a well-established mechanism for POP [6–8]; however, not all women with levator tears develop POP [7]. The vagina, a fibromuscular tissue, is suggested to play a role in providing pelvic organ support [5,9,10]. Vaginal smooth muscle (VaSM) organization [11,12], composition [11] and maximum muscle tone [13] are decreased in prolapsed tissues. Further, current surgical interventions using meshes to increase pelvic support negatively affect vaginal muscle tone [14].
Maximum and basal (resting) muscle tone are key characteristics of both striated and smooth muscle cells. The dominant clinical metric for assessing levator ani function is contractile strength or maximum muscle tone [15]. Basal tone, however, is negatively associated with POP, indicating its importance [16]. Similarly, maximum tone of the vaginal wall is primarily investigated [13,14,17–21], but the VaSM basal contribution may also be of significance [22–24]. Along the circumferential axis, maximum tone is greater in the proximal than the distal region of the vaginal wall [18]. Regional variation may arise from different embryological origins; the proximal vagina derives from the Müllerian ducts and the distal from the urogenital sinus [25,26].
Vaginal maximum tone was previously investigated by isometric contractile testing under a fixed extension on tissue strips taken along the circumferential [14,18] or axial direction [13,17,19,20]. The vaginal wall, however, is subjected to multiaxial loading within the body as a result of intra-abdominal pressures. Biaxial contractile testing permits application of multiaxial loads to better replicate physiological conditions [21,27]. Further, biaxial testing permits simultaneous quantification of muscle tone along the circumferential and axial axes [21]. Previously, planar biaxial testing was implemented to investigate smooth muscle maximum contribution to the mechanical behaviour of the rat vaginal wall [21]. In addition, extension–inflation protocols were leveraged to assess the contractile response of the porcine and murine arteries under isobaric–axially isometric contraction [27–29]. This methodology permits preservation of tissue geometry and smooth muscle–matrix interactions. Extension–inflation protocols have not been performed on murine vaginal tissue to assess muscle function. Rodent models are used to investigate biomechanical properties [30–34] and contractile function [17,18,20,21] of vaginal tissue. However, although not a substitute for human tissue, similarities in the gross connective vaginal tissue anatomy are reported [32] and may provide insight into structure–function relationships.
Currently, it is not understood if VaSM dysfunction contributes to the pathogenesis of POP [35]. A lack in understanding may result from limited investigation of smooth muscle basal and maximum contribution to the biaxial mechanical response of vaginal tissue. Therefore, the objectives for this study are to (i) determine smooth muscle basal contribution to the biaxial mechanical behaviour of the proximal and distal vagina and (ii) assess the regional contractile response under various circumferential and axial loads. We hypothesize that basal tone results in a decrease in outer diameter and material stiffness, and that the proximal region is materially stiffer than the distal. As for maximum contraction, we hypothesize that higher loads (pressure and length) decrease the contractile response, and that the proximal region generates a greater contractile response than the distal.
2. Methods
Non-parous four- to six-month-old female C57BL/6 mice (24.4 ± 1.8 g; mean±s.d.) (Jackson Laboratory, Bar Harbor, ME, USA, and Charles River, Wilmington, MA, USA) at oestrus were used throughout this study. Animals were housed under standard conditions (12 h light/dark cycles) and all procedures were approved by the Institute Animal Care and Use Committee at Tulane University, New Orleans, LA, USA. A pilot study (Jackson Laboratory (n = 3) and Charles River (n = 3)) revealed no significant differences in the mechanical properties of vaginal tissue between vendors. For functional and microstructural assessment, 29 animals were euthanized by guillotine without anaesthesia or carbon dioxide (CO2) as outlined in figure 1. Upon sacrifice, the reproductive system was dissected immediately and placed in cold (4°C) Hank's balanced salt solution, followed by separation of the vagina from the cervovaginal complex as illustrated previously by Robison et al. in figures 1 and 2 [33].
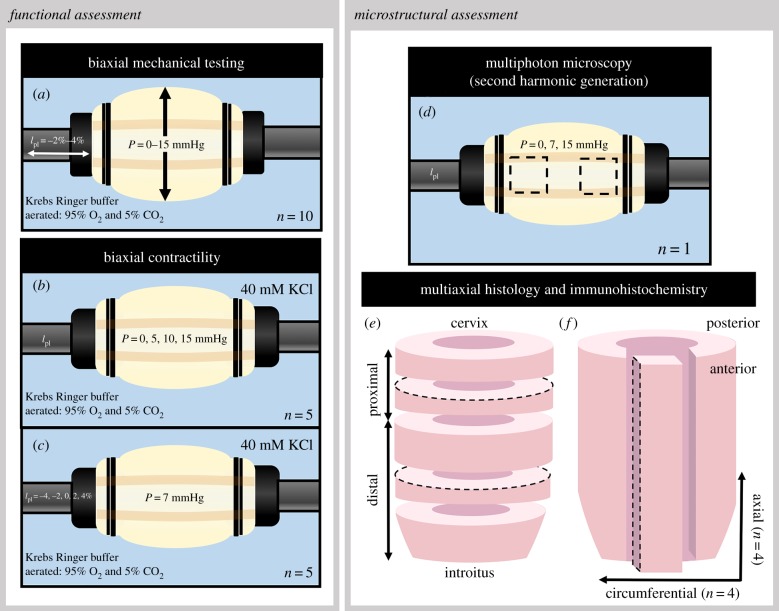
Figure 1.
Schematic for functional and microstructural assessment of murine vaginal tissue. Tissues were subjected to extension–inflation protocols (a) for biaxial mechanical testing. Biaxial contractile testing was executed under various constant pressures (b) and fixed lengths (c). Multiphoton microscopy with second harmonic generation was performed at the averaged physiological length along the anterior wall at the proximal and distal regions under three constant pressures (d). Histological and immunohistochemical analysis was performed on circumferential sections taken from the proximal and distal regions of the vaginal wall (e); axial sections were taken along the length of the anterior wall (f). The dashed lines represent the region of interest. (Online version in colour.)
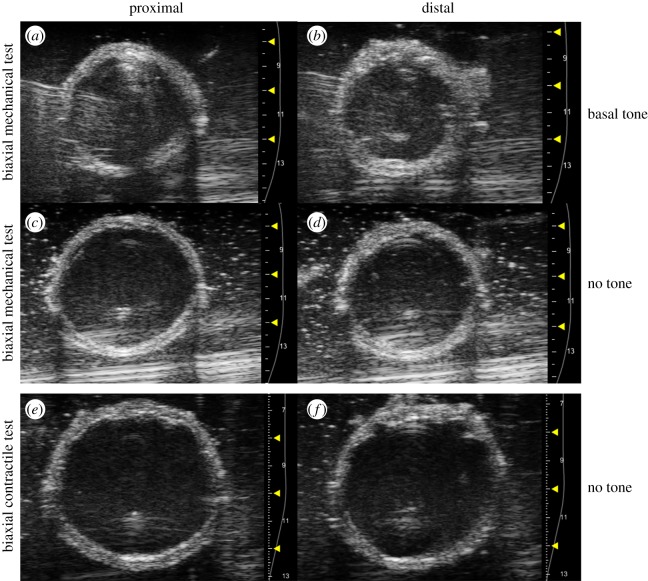
Figure 2.
Representative ultrasound B-mode images acquired at the proximal (a,c,e) and distal (b,d,f) regions of the vaginal wall. During biaxial mechanical testing (a–d), images were acquired at the unloaded configuration with (a,b) and without (c,d) basal tone. For biaxial contractile testing (e,f), images were acquired at the unloaded configuration without muscle tone. (Online version in colour.)
2.1. Biaxial mechanical testing
Extension–inflation testing was used to assess biaxial mechanical properties (figure 1a; n = 10), which permitted maintenance of native smooth muscle cell–matrix interactions and tissue geometry [33,34,36–38]. Freshly isolated vaginal tissue was mounted within a pressure myograph system (Danish MyoTechnologies, Aarhus, Denmark) onto 3.75 mm diameter cannulas with two 6-0 sutures. The bath was filled with Krebs Ringer buffer (KRB; 120 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 11 mM glucose) at 37°C aerated with 95% O2 and 5% CO2 to maintain pH at 7.4 for smooth muscle cell viability [27,39]. Custom acrylic housing prevented aeration interference with force measurements and outer diameter tracking [40]. The transducer-measured force and outer diameters were recorded via an integrated system of components (Eclipse TS100 video-microscope; Nikon, Melville, NY, USA) and software (Myoview Software; Danish MyoTechnologies, Aarhus, Denmark). The outer diameter for the proximal and distal regions was tracked 1.5 mm from the suture to minimize experimental variability due to end effects. This value was selected owing to the proximal region ranging from 1.5 to 2.0 mm in length.
The unloaded configuration was defined as when the tissue was slightly buckled and not collapsed (3 mmHg) [33,34]. Post explant, the vaginal tissue retracted in length [33,34,38]; therefore, in order to quantify tissue properties under near physiologically relevant loads, the tissue was axially extended [33,34,37,38,41] to an estimated physiological length (1.15 times the unloaded length) [33,34]. Initial preconditioning was performed to minimize hysteresis with cyclic pressurization from 0 mmHg to the mean measured in vivo pressure (7 mmHg) over five cycles at 1.5 mmHg s−1 [33,42]. The mean measured in vivo pressure was determined by balloon catheterization as described and reported in the electronic supplementary material, figure S1. The physiological length under basal tone was identified where the transducer-measured forces held constant over a range of increasing physiological pressures [33,34,37,38,41]. Preconditioning with five cycles of pressurization (0–15 mmHg) at the physiological length, and axial extension (10 µm s−1) from 2% below to 2% above the physiological length at a constant pressure (5 mmHg) was performed [33,34]. The tissue equilibrated for 10 min at the physiological length under 5 mmHg, followed by re-establishment of the unloaded configuration [33].
The vaginal tissue was subjected to pressure–diameter testing (0–15 mmHg) over five cycles at and about (−2%, +2% and +4%) the physiologic length [33,34,37]. This was followed by force–length testing to confirm the physiological length [33,34,37,41]. The tissue was axially extended from 2% below to 2% above the physiological length under four constant pressures: a tare load (P = 2 mmHg), one-third maximum pressure (P = 5 mmHg), two-thirds maximum pressure (P = 10 mmHg) and maximum pressure (P = 15 mmHg). Smooth muscle tone was removed by replacing the circulating medium with calcium-free KRB supplemented with 2 mM of a calcium-chelating agent, egtazic acid, and incubated for 30 min [37,43]. Pressure–diameter tests were repeated at the relaxed (no tone) state under the same axial lengths as the basal state. Force–length tests were performed 2% above and below the physiological length without smooth muscle tone.
2.2. Biaxial contractile testing
The evaluation of the biaxial contractile response with isobaric–axially isometric contraction under physiologically relevant loads (figure 1b,c) was performed with a pressure myograph system [27,28,44]. Vaginal tissue was mounted and the unloaded configuration was determined, followed by initial preconditioning and identification of the physiological length. For pressure-dependent contractility (figure 1b; n = 5), the tissue was preconditioned with five cycles of pressurization (0–15 mmHg), followed by re-establishing the unloaded configuration [33]. To maintain viability of the VaSM, preconditioning with maximum contraction was performed by stimulating the vaginal tissue under low loading conditions [27,28]. The intraluminal pressure was set to 5 mmHg and the vaginal tissue was axially stretched to a length from the unloaded length where the transducer-measured force equalled zero; this was followed by stimulation with 40 mM KCl for 5 min until a steady response was achieved. This concentration induced maximal changes in force and the outer diameter was determined by a pilot study (n = 5). Following stimulation, the tissue was washed twice with KRB in order to return to basal tone. This was repeated at 9 mmHg, one standard deviation above the mean measured in vivo pressure [27,28]. The tissue was returned to its physiological length for equilibration. After equilibration, the intraluminal pressure was set to 0 mmHg and contracted with 40 mM KCl then washed twice to return to basal tone. This was repeated to assess contraction as a function of intraluminal pressure at three constant pressures: 5, 10 and 15 mmHg.
To assess contraction as a function of axial length (figure 1c; n = 5), a separate cohort of animals was used owing to the inability to restore basal outer diameter and force values after being contracted under higher circumferential and axial loads. It was imperative to obtain accurate stress values to determine the relationship between the stress and stretch response. Sample preparation and experimental set-up were conducted in the same manner; however, preconditioning was performed with five cycles of axial extension (10 µm s−1) from 4% below to 4% above the physiological length under a constant pressure (5 mmHg). Following smooth muscle preconditioning, contraction as a function of axial length was assessed. Pressure was held constant at the mean measured in vivo pressure (7 mmHg), and the tissue was contracted at 4% and 2% below, above and at the physiological length [27,33,34]. After contractile assessment, the passive state was induced and tissue geometry and force values were recorded [27,44].
2.3. Ultrasound imaging for vaginal wall thickness
Vaginal wall thickness was determined from ultrasound images acquired at the unloaded configuration for each state using the Vevo2100 ultrasound imaging system (FUJIFILM VisualSonics, Inc., Toronto, ON, Canada) [45]. Short-axis B-mode ultrasound images were obtained at each region of interest with a 40 MHz centre frequency transducer (LZ550) under basal tone and at the relaxed state for biaxial mechanical testing and the relaxed state for biaxial contractile testing (figure 2). The thickness of the proximal and distal vagina were determined in ImageJ (NIH, Bethesda, MD, USA).
2.4. Mechanical testing and contractility data analysis
The unloaded volume was calculated at the proximal and distal region from the video-microscope unloaded outer radius, Ro, ultrasound unloaded thickness, H, and unloaded length, L, with basal tone and when relaxed. The unloaded regional length for the proximal and distal region was one-third and two-thirds of the total length, respectively, owing to different embryological origins [25,26]. Assuming conservation of volume (i.e. incompressibility), the unloaded volume, regional deformed outer radius, ro, and length, l, were used to calculate the deformed inner radius (ri)
| 2.1 |
| 2.2 |
The wall-averaged circumferential (θ), axial (z) and radial (r) Cauchy stresses (σ) were determined, where P is the intraluminal pressure and Ft is the transducer-measured force [37,46]
| 2.3 |
| 2.4 |
| 2.5 |
The change in stress with contraction (Δσ) was calculated by subtracting the passive stress where the VaSM was relaxed (σrelaxed) from the total stress where the VaSM was maximally contracted (σcontracted) [27,42,47,48]
| 2.6 |
and
| 2.7 |
Circumferential stretch ratio (λθ) was defined by the radius at the mid-vaginal wall of the deformed state to the undeformed state [37]. The axial stretch ratio (λz) was calculated as the axial length with respect to the relaxed unloaded axial length
| 2.8 |
and
| 2.9 |
Compliance and tangent moduli were determined by taking the slope of the pressure–diameter and stress–strain curve within the physiological pressure range (7 ± 2 mmHg; mean ± s.d.) at the physiological length [49].
2.5. Multiphoton microscopy
The collagen fibre architecture of the anterior vaginal wall (n = 1) was visualized via second harmonic generation (SHG) at the average physiological length (1.15 times the unloaded) under varied constant pressures (0, 7, 15 mmHg; figure 1d). As in experimental studies, a tare pressure of 3 mmHg was applied. The tissue was imaged with a Zeiss 710 NLO inverted confocal microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA) in combination with a mode-locked Ti : sapphire Chameleon Ultra laser (Coherent Inc., Santa Clara, CA, USA) equipped with a non-descanned detector. Laser excitation was generated at 800 nm and the backward SHG signal was collected in the 380–430 nm range with a 40× oil immersion objective. Images were collected with the ZEN imaging software (Carl Zeiss Microscopy GmbH) at a resolution of 0.35 × 0.35 µm2 per pixel at an 8 bit pixel depth. Under each loading condition at the proximal and distal regions, three or four images were collected at a depth ranging between 7 and 10 µm from the adventitia. The collagen fibre angular distribution and alignment were quantified with an open source software, CurveAlign (LOCI, Madison, WI, USA; http://www.loci.wisc.edu/software/curvealign).
2.6. Multiaxial histology and immunohistochemistry
Specimens (n = 8) were fixed with 10% formalin for 24 h then paraffin embedded. Circumferential sections of 4 µm thickness (n = 4) were taken at the proximal and distal regions (figure 1e); axial sections of 4 µm thickness (n = 4) were taken along the anterior wall (figure 1f). For histological assessment, sections were stained with Masson's trichrome and Hart's elastin stain. Antigen retrieval for immunohistochemical analysis of α-smooth muscle actin (α-SMA) (Biocare, Pacheco, CA, USA) was performed for 15 min (pH = 6.0). Sections were blocked for 5 min in hydrogen peroxide and endogenous mouse immunoglobulin G (Biocare, Pacheco, CA, USA) for 10 min. The sections were incubated for 30 min with the primary antibody (1 : 200) and detected with the MACH 4 mouse probe (10 min) and rabbit polymer (10 min) (Biocare, Pacheco, CA, USA). Colour was developed by incubating in 3,3′-diaminobenzidine substrate for 5 min, followed by counterstaining with haematoxylin.
Brightfield images were taken using an Olympus BX51 microscope, an Olympus DP27 Digital Camera and cellSens™ software (Olympus Corporation, Center Valley, PA, USA) at 4× and 20× magnification. Area fraction quantification was performed on 4× images displaying the full cross-section with Colour Deconvolution, an open source plug-in for ImageJ, and a custom protocol using GNU Image Manipulation Program as previously described [34,50,51]. The epithelium, which is suggested to be non-load bearing, was excluded from this calculation [34,46].
2.7. Statistical analysis
To evaluate the smooth muscle basal contribution to structural and material properties, paired t-tests were conducted on the unloaded thickness, volume, physiological length, physiological outer diameter, compliance and tangent modulus comparing basal tone and no tone at each region. Differences in circumferential and axial tangent modulus were evaluated with paired t-tests. Regional differences were further evaluated with paired t-tests. For assessment of the contractile response with loading, a one-way ANOVA with the Tukey post hoc test was performed. The level for statistical significance was set at p ≤ 0.05. A two-way ANOVA evaluated the effects of region and orientation on the change in stress with contraction and area fraction, followed by a post hoc t-test and Bonferroni correction (p < 0.05/2) when necessary. All statistical analyses were performed in R (R Statistical Software, Vienna, Austria). The results are reported as mean ± standard error of the mean (s.e.m.). All experimental data were used in the data analysis.
3. Results
3.1. Basal smooth muscle contribution to vaginal wall mechanical properties
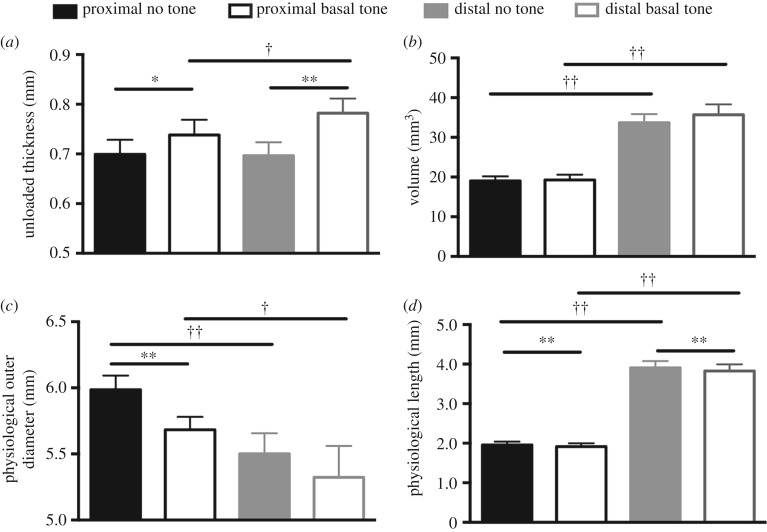
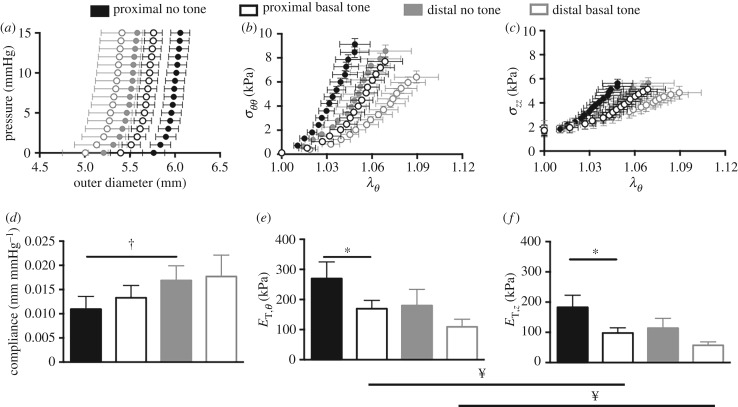
Ultrasound imaging revealed a significant increase in unloaded thickness with basal tone compared with no tone for the proximal (p < 0.05) and distal (p < 0.001) regions (figure 3a). In addition, under basal tone the unloaded thickness for the distal region was greater than that for the proximal region (p < 0.05). Although the unloaded volume with basal and no tone was greater at the distal region than at the proximal region (p < 0.001), significance was not identified between the two states at either region (figure 3b). The physiological outer diameter that was measured at the physiological length and mean pressure (7 mmHg) displayed a significant decrease in the proximal region under basal tone (p < 0.001). Further, the physiological outer diameter was significantly smaller for the distal region than for the proximal region with (p < 0.05) and without (p < 0.001) basal tone. The physiological length was significantly shorter (p < 0.001) for the proximal and distal regions with the presence of basal tone (figure 3d). Basal tone induced a leftward shift in the pressure–diameter curve, denoting a decrease in outer diameter compared with no tone (figure 4a). Basal tone induced a rightward shift in the stress–stretch curve, denoting an increase in distensibility (figure 4b,c). Without basal tone, the distal region was more compliant (p < 0.05) than the proximal region (figure 4d). At the proximal region, basal tone resulted in a significant decrease (p < 0.05) in circumferential (figure 4e) and axial (figure 4f) tangent moduli. With basal tone, the circumferential tangent moduli was significantly larger than the axial for the proximal and distal regions (p < 0.05).
Figure 3.
Basal tone resulted in a significant increase in the unloaded thickness at the proximal (black) and distal (grey) regions with the distal region being thicker than the proximal (a). The volume of the vagina was larger for the distal region than for the proximal, with (open) and without (filled) basal tone (b). Basal tone induced a significant decrease in outer diameter in the proximal region (c). The proximal region presented a larger outer diameter than the distal, with and without basal tone. There was a decrease in physiological length with basal tone for the proximal and distal regions (d). Data are reported as mean ± s.e.m. Statistical significance is denoted by state *p < 0.05 and **p < 0.01, as well as location †p < 0.05 and ††p < 0.01.
Figure 4.
Vaginal wall outer diameter for the proximal (black) and distal (grey) regions as a function of increasing intraluminal pressure. Basal tone (open) resulted in a leftward shift in the pressure–diameter curve with respect to no tone (filled) denoting decreased vaginal wall diameter (a). Circumferential (b) and axial (c) stresses at the physiological length versus circumferential stretch. The presence of basal tone resulted in a rightward shift, denoting an increase in distensibilty. The absence of basal tone resulted in a more compliant distal region than proximal (d). Basal tone decreased the circumferential (e) and axial (f) tangent moduli at the proximal region. With basal tone, the circumferential tangent moduli were larger than the axial for both regions. Data are reported as mean ± s.e.m. Statistical significance (p < 0.05) is denoted by state *, location † and orientation ¥.
3.2. Vaginal contractile response to circumferential and axial loading
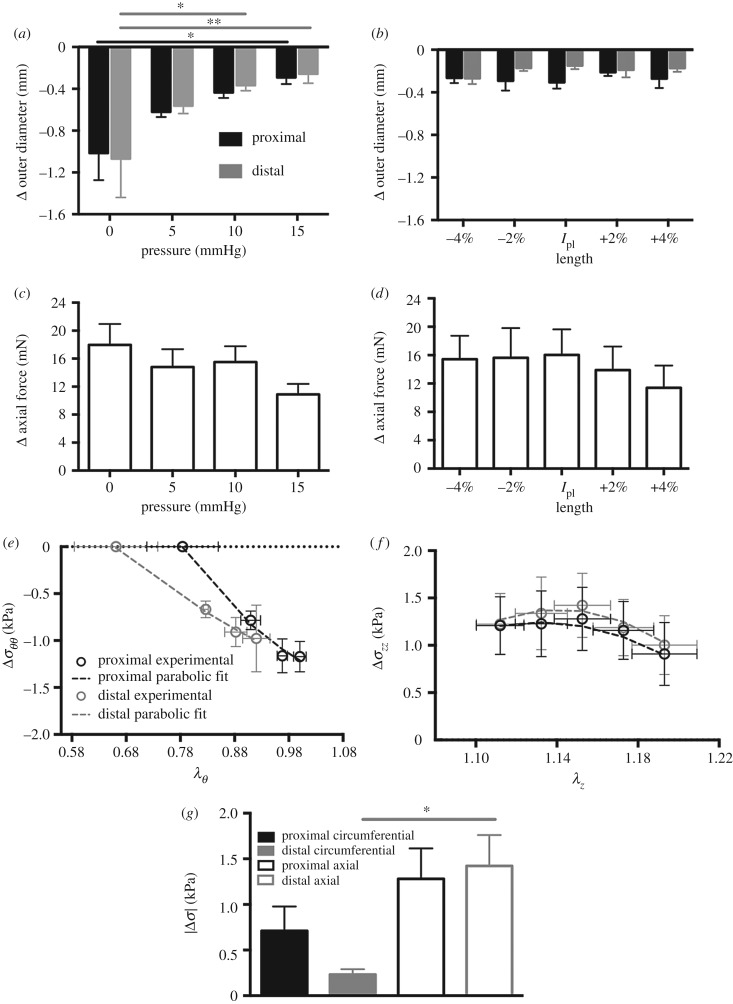
Stimulation with 40 mM KCl induced a tonic contraction resulting in a decrease in outer diameter and increase in axial force with reference to the relaxed state (figure 5a–d). The one-way ANOVA identified significant differences in the change in outer diameter with respect to intraluminal pressure for the proximal (p < 0.05) and distal (p < 0.01) regions (figure 5a). The change in diameter was reduced in the proximal (p < 0.05) and distal (p < 0.01) regions between 0 and 15 mmHg. Further, the change in outer diameter with contraction was reduced (p < 0.05) at the distal region between 0 and 10 mmHg. The one-way ANOVA did not identify statistically significant differences in the change in outer diameter as a function of the change in axial length (figure 5b), nor the change in axial force as a function of the pressure or axial length (figure 5c,d). Contraction resulted in a decrease in circumferential stress and increase in axial stress (figure 5e,f). A parabolic relationship (Δσ = a(λ − h)2 + k) was observed for the change in circumferential stress to circumferential stretch (RMSE; proximal = 0.09 and distal = 0.04; figure 5e) and axial stress to axial stretch (RMSE; proximal = 0.09 and distal = 0.08; figure 5f). The two-way ANOVA identified statistically significant differences (p < 0.001) with respect to orientation of the change in stress with contraction (figure 5g). At the distal region, the absolute value of axial stress was significantly higher (p < 0.001) than that of circumferential stress. As for the proximal region, a non-significant increase in axial stress (p < 0.1) was observed compared with circumferential stress.
Figure 5.
Change in outer diameter with contraction under various physiologically relevant pressures at the fixed physiological length. The magnitude of the change in diameter with contraction was diminished at 15 mmHg for the proximal (black) and distal (grey) region, and 10 mmHg for the distal region only (a). Change in outer diameter with contraction at various physiologically relevant lengths under the mean measured in vivo pressure (b). Change in axial force with contraction under various physiologically relevant pressures at the fixed physiological length (c). Change in axial force with contraction at various physiologically relevant lengths under the mean measured in vivo pressure (d). A parabolic function (dashed lines) describes the changes in circumferential stress to circumferential stretch (RMSE; proximal = 0.09 and distal = 0.04) (e) and axial stress to axial stretch (RMSE; proximal = 0.09 and distal = 0.08) (f) curves. The absolute value of the change in stress at the physiological length and pressure. At the distal region, axial stress was greater than circumferential stress (g). Data are reported as mean ± s.e.m. Statistical significance denoted by *p < 0.05 and **p < 0.01.
3.3. Collagen fibre architecture under loading
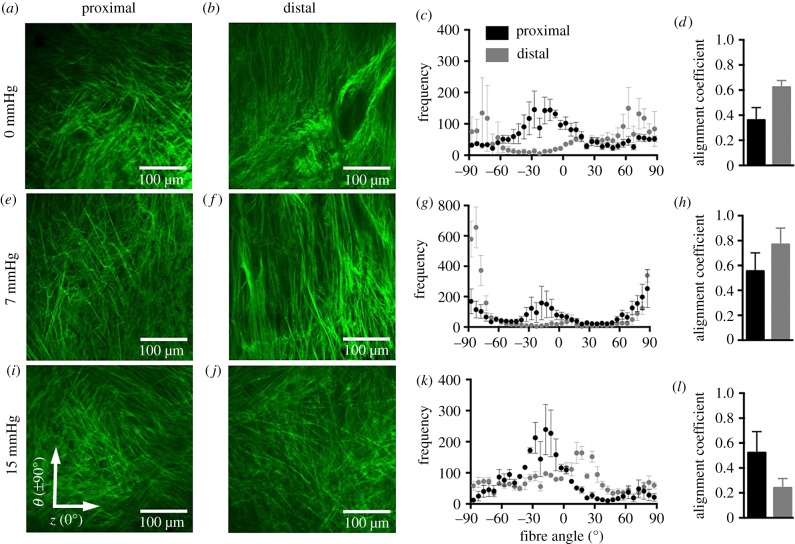
Imaging of the collagen fibre architecture qualitatively and quantitatively demonstrated that, at 0 and 7 mmHg, the proximal and distal regions exhibited marked differences in collagen fibre dispersion (figure 6a–h). At the distal region under 0 and 7 mmHg, collagen fibres displayed greater alignment towards the circumferential axis (±90°; figure 6b,c,f,g). The proximal region under 0 mmHg exhibited collagen fibres oriented towards the diagonal axis (30°; figure 6a,c); however, under 7 mmHg, fibres reoriented towards the circumferential axis (±90°; figure 6e,g). The proximal and distal regions were more aligned near the diagonal axis (±30°) towards the axial direction under 15 mmHg (figure 6i–k). The distal region presented a larger alignment coefficient, denoting that the distal region was more uniformly aligned towards a preferred direction than the proximal region (figure 6d,h).
Figure 6.
Multiphoton microscopy with SHG of the collagen fibre architecture at the proximal (a,e,i) and distal (b,f,j) regions of the anterior vaginal wall under 0 (a,b), 7 (e,f) and 15 (i,j) mmHg at the average physiological length. The frequency of the number of individual fibre segments with its respective orientation is reported with 0° denoting the axial axis and ±90° denoting the circumferential (c,g,k) at increments of 5°. At 0 and 7 mmHg, collagen fibres are oriented towards the circumferential axis at the distal region. At the proximal region, fibres are oriented towards the circumferential and diagonal axes (c,g). Under 15 mmHg, at the proximal and distal regions, the fibres are oriented towards the axial direction (k). The alignment coefficient describes the collagen fibre orientation towards a preferred direction, with 1 being highly aligned (d,h,l). The alignment coefficient was larger for the distal region than for the proximal at 0 and 7 mmHg. Data were averaged over several images and are reported as mean ± s.e.m. Imaging depth was 7–10 µm from the adventitial layer or outer wall. (Online version in colour.)
3.4. Regional multiaxial histological and immunohistochemical analysis
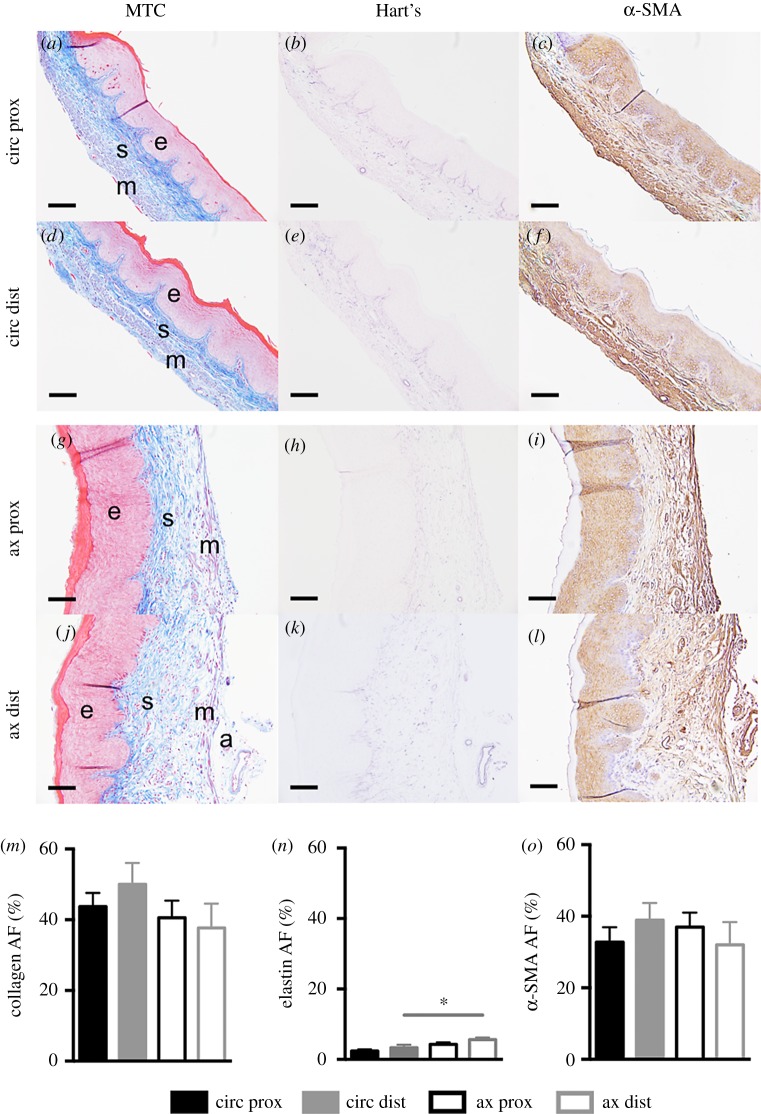
The two-way ANOVA identified statistically significant differences (p < 0.01) with respect to orientation in the elastin area fraction. At the distal region, the elastin area fraction was greater (p < 0.01) for axial sections (5.6 ± 0.6%) than for circumferential sections (3.3 ± 0.6%) (figure 7n). The effect of region and orientation, however, were not found to be significant for collagen and α-SMA (figure 7m,o).
Figure 7.
Representative histological images were acquired at 20× magnification. Proximal (prox; a–c, g–i) and distal (dist; d–f, j–l), circumferential (circ; a–f) and axial (ax; g–l) sections stained with Masson's trichrome (MTC; a,d,g,j), Hart's elastin (b,e,h,k) and α-SMA (c,f,i,l). Layers of the vaginal wall are denoted: epithelium (e), subepithelium (s), muscularis (m) and adventitia (a). Area fraction (AF) for collagen (m), elastin (n) and α-SMA (o) are reported for the proximal (black) and distal (grey) regions along the circumferential (close) (filled) and axial (open) sections. At the distal region, the elastin area fraction was greater for the axial section than for the circumferential. Data are reported as mean ± s.e.m. Statistical significance is denoted by *p < 0.05/2. Scale, 100 µm. (Online version in colour.)
3.5. Contractility following elastase digestion
This study demonstrated that, under maximum contraction, the distal region axial stress was greater than the circumferential stress (figure 5g). Additionally, the elastin area fraction was greater along the axial axis than along the circumferential axis (figure 7n). Prior work shows that a decrease in elastin reduces the contractile response [27]. Therefore, a pilot study (n = 5) was conducted to investigate the maximum contraction of the vaginal wall following elastase digestion. Experimental methods were performed as described in §2.2. Maximum contraction was performed at the physiological length and mean pressure (7 mmHg), before and after elastase digestion. The vaginal tissue was treated with 15 U of elastase for 45 min as described previously [34]. Elastase digestion significantly decreased (p < 0.05) the change in axial force with contraction (electronic supplementary material, figure S2B).
4. Discussion
This study presents the contribution of smooth muscle to the biaxial mechanical behaviour of the murine vaginal wall. The vaginal wall contains smooth muscle cells oriented primarily along the circumferential and axial axes. Further, the vaginal wall is subjected to multiaxial loading within the body. Therefore, within this study, extension–inflation protocols were used to assess the basal smooth muscle tone contribution to the mechanical function of the vagina as well as to investigate the maximum tone under various circumferential and axial loads. This permitted simultaneous characterization of the smooth muscle contribution along the circumferential and axial axes. Smooth muscle basal and maximum tone significantly contributed to the biaxial mechanical behaviour of the murine vaginal wall.
Smooth muscle basal tone significantly decreased vaginal length and outer diameter (figure 3c,d). These results coincide with the changes in vaginal wall geometry induced by smooth muscle for sexual function [52,53]. An early physiological event of sexual arousal is characterized by pelvic nerve stimulation followed by smooth muscle relaxation. This induces an increase in vaginal length and diameter to permit penetration [52,53]. Further, basal tone significantly decreased material stiffness in the proximal region of the vagina (figure 4e,f). Material stiffness decreased with increased muscle tone (basal and maximum) in the rabbit basilar artery [23]. Kelly & Chowienczyk [54] hypothesized that a decrease in muscle tone transfers loads to other components in the arterial wall, such as collagen fibres, increasing stiffness of the tissue. This transfer of load may result in a decrease in collagen fibre undulation, thus increasing material stiffness. Therefore, we hypothesize that basal tone induces a transfer of load from collagen and elastic fibres to the smooth muscle cells, increasing collagen fibre undulation and thereby decreasing material stiffness of the vaginal wall.
Anisotropy, i.e. the direction-dependent mechanical properties, of vaginal tissue was investigated by comparing the circumferential and axial tangent moduli. This study demonstrated that, with basal tone, the circumferential direction was materially stiffer than the axial. Within this and a prior study, statistically significant differences in passive (no muscle tone) material stiffness between the circumferential and axial direction were not detected near the physiologically relevant configuration (length/axial stretch and pressure) [33]. In the previous study, anisotropy of the passive vagina was dependent on loading protocol [33]. This study, however, demonstrates that smooth muscle tone significantly contributes to the direction-dependent properties near physiologically relevant conditions. Investigation of anisotropy is useful for understanding disease progression. For example, the development of abdominal aortic aneurysms results in an increase in anisotropy, with a preference of material stiffening in the circumferential direction [55]. Understanding the vaginal wall direction-dependent mechanical response may be useful for better understanding the development of POP.
In addition to the smooth muscle basal contribution, the maximum contribution was investigated as a function of mechanical loading. Maximum tone in the circumferential direction significantly decreased with increased intraluminal pressure (figure 5a). This response indicates that VaSM is sensitive to mechanical loading by pressure. This may be supported by prior observations that increased pressure results in arterial wall adaptation mediated by smooth muscle cells in order to maintain a preferred homeostatic stress and restore material properties [22,56,57]. Perturbations to the mechanical environment and effect on vaginal function, however, are not defined. The vagina spends a majority of the time working within a mechanical environment under non-pregnant conditions. During pregnancy, which is a brief period of time over a woman's lifespan, the vaginal canal is subjected to extreme mechanical conditions that require vaginal remodelling to permit passage of the foetus [17,58–60]. Although the vagina is able to withstand instantaneous changes in pressure outside of pregnancy [61], increased mechanical loading due to increased intra-abdominal pressure (i.e. chronic cough, constipation, repeated heavy lifting) is a risk factor for POP [62]. Therefore, the effect of sustained increased mechanical pressure on smooth muscle-regulated mechano-mediated adaptation merits further investigation in vaginal tissue.
For the murine vagina, a parabolic function described the regional stress–stretch curve behaviour under maximum contraction reasonably well (figure 5e,f). A parabolic relationship between active stress and stretch under isometric contraction is reasonable for the vasculature [42,47,48] and the rat vagina [21]. It was reported that the deformed state of the arterial wall alters the configuration of contractile proteins inside the smooth muscle cell, dictating the active response [63]. Therefore, in addition to mechanical load, the change in stress with contraction depends on deformation (stretch).
The change in circumferential stress with contraction was not significantly different between the proximal and distal regions (figure 5g). This finding corresponds to rat vaginal tissue, wherein regional differences in maximum contraction along the circumferential axis were not detected in response to KCl [18]. The absolute value of the change in axial stress was significantly higher than circumferential stress at the distal region (figure 5g). This result is similar to the biaxial behaviour of the rat vagina. Stimulation with KCl induced a greater contractile response in the axial direction than in the circumferential direction, by generating a higher active stress [21]. For the murine vagina, this result may be described by VaSM interaction with elastic fibres. At the distal region, the elastin area fraction was greater on the axial axis than on the circumferential axis (figure 7n). The pilot study (n = 5) demonstrated that removal of elastin significantly decreased the axial force with contraction (electronic supplementary material, figure S2B). This corresponds to vasculature where the contractile response of the aorta from elastoplastic mice is decreased [27]. Therefore, the larger elastin area fraction along the axial axis of the murine vagina may result in a greater contractile response in the axial direction than in the circumferential direction.
Additional microstructural analysis corresponded with mechanical findings. Regional differences in mechanical properties (figure 4) as well as the collagen and elastic fibre area fraction were not identified (figure 7m,n). In addition to the composition of collagen and elastin [34,64–66], the organization of the fibres may dictate the mechanical response [67–69]. Although collagen fibres in the distal region were initially more highly aligned towards the circumferential axis (figure 6a–h), the tissue was not materially stiffer than that in the proximal region. This may result from imaging being performed between the adventitia and muscularis; however, collagen density is greater in the subepithelial layer (figure 7a,d,g,j). Additional analysis is needed throughout the thickness of the vaginal wall. Nevertheless, this study provides initial data on collagen fibre organization in the murine vagina, which is currently unknown. Lastly, regional differences in α-SMA were not detected. This corresponded to not observing regional differences in maximum contraction in response to KCl. Directional differences in maximum contraction were detected; however, differences in α-SMA were not identified. In addition to elastic fibres, other smooth muscle contractile proteins may be of significance [70,71].
This study is not without limitations. First, the vaginal wall was assumed to be incompressible. Although volume change between the basal and passive state was not statistically significant, further investigation of isochoric motion is needed [27]. This study provides relevant data demonstrating the smooth muscle contribution to the biaxial mechanical behaviour of murine vaginal tissue. Further, end effects (Saint-Venant's principle) were assumed to be negligible, although the aspect ratio of the murine vagina is nearly 1 : 1. Regardless, this study provides information on the regional behaviour where the proximal region is comparable to the distal. Experimental variability between the regions due to end effects were minimized by tracking the same distance away from the cannula. Alternatively, the cervovaginal complex could remain intact, increasing the aspect ratio to minimize end effects. This, however, may induce additional artefacts in the force and diameter measurements owing to murine cervical smooth muscle consisting of brief periods of both relaxation and contraction [39]. Therefore, coupling the cervical phasic behaviour to reduce end effects will lead to difficulty in independently evaluating vaginal wall basal and maximum tone.
5. Conclusion
Prior work focused on characterizing the passive uniaxial properties of vaginal tissue [72–76]. Although this has provided initial valuable information to the field, it does not recapitulate the multiaxial loading the tissue experiences in vivo. Further, it does not account for the direction-dependent mechanical behaviour. In addition, the passive behaviour does not consider active smooth muscle interaction with the extracellular matrix and its contribution to the mechanical properties. Recent work, however, highlights the significance of the biaxial properties and smooth muscle contribution to vaginal function [21,33,34]. This study investigated smooth muscle basal and maximum contribution to the proximal and distal regions of the murine vaginal wall. Basal smooth muscle tone significantly contributed to the biaxial mechanical properties of the murine vagina. Further, the magnitude of circumferential contraction was significantly decreased with increased pressure and was greater in the axial direction. The present findings suggest that vaginal active smooth muscle significantly interacts with collagen and elastin, contributing to the biaxial mechanical behaviour. Therefore, smooth muscle tone along the circumferential and axial axes should be evaluated when characterizing the mechanical properties of vaginal tissue. In addition to changes in collagen [77] and elastin [78] with prolapse, changes in smooth muscle composition and phenotype [11–13] may occur, altering the mechanical behaviour of the tissue. Accounting for smooth muscle tone may aid in better understanding the functional changes that occur with prolapse progression. Ultimately, this may assist in further elucidating the underlying mechanisms for prolapse, in order to improve current preventative and treatment strategies.
Supplementary Material
Acknowledgements
We would like to acknowledge Sae-Il Murtada, PhD (Yale University), for training on the biaxial contractile protocol. Further, we acknowledge Mary K. Clark and Monica Hanon (Georgia Institute of Technology) for assistance with multiphoton microscopy.
Data accessibility
Data available from the Dryad Digital Repository [79].
Authors' contributions
G.L.C. carried out the experimental design, mechanical and contractile test, data analysis and drafting of the manuscript; A.P.P.-P. carried out multiphoton imaging, data analysis and drafting of the manuscript; D.J.L. assisted in ultrasound imaging and drafting of the manuscript; K.S.M. participated in the study design and drafting of the manuscript; all other authors (L.D., L.R.K., S.H.L., C.L.B., R.L.G.) contributed by scientific discussion and drafting of the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed herein.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by NSF Early Faculty CAREER Development Award CMMI-1751050 (K.S.M.); NSF CMMI-1727573 (R.L.G.); NIH HL133619 (S.H.L.); and the Louisiana Board of Regents Fellowship (G.L.C.).
References
- 1.Wu JM, Matthews CA, Conover MM, Pate V, Funk MJ. 2014. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet. Gynecol. 123, 1201–1206. ( 10.1097/AOG.0000000000000286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. 2001. Cost of pelvic organ prolapse surgery in the United States. Obstet. Gynecol. 98, 646–651. ( 10.1097/00006250-200110000-00021) [DOI] [PubMed] [Google Scholar]
- 3.Mant J, Painter R, Vessey M. 1997. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br. J. Obstet. Gynaecol. 104, 579–585. ( 10.1111/j.1471-0528.1997.tb11536.x) [DOI] [PubMed] [Google Scholar]
- 4.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. 2002. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am. J. Obstet. Gynecol. 186, 1160–1166. ( 10.1067/mob.2002.123819) [DOI] [PubMed] [Google Scholar]
- 5.Delancey JOL. 1992. Anatomic aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 166, 1717–1728. ( 10.1016/0002-9378(92)91562-O) [DOI] [PubMed] [Google Scholar]
- 6.Benson T, Weidner AC. 2000. Pelvic muscle electromyography of levator ani and external anal sphincter in nulliparous women and women with pelvic floor dysfunction—discussion. Am. J. Obstet. Gynecol. 183, 1390–1401. ( 10.1067/mob.2000.111073) [DOI] [PubMed] [Google Scholar]
- 7.DeLancey JOL, et al. 2007. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet. Gynecol. 109, 295–302. ( 10.1097/01.AOG.0000250901.57095.ba) [DOI] [PubMed] [Google Scholar]
- 8.Friedman S, Blomquist JL, Nugent JM, McDermott KC, Munoz A, Handa VL. 2012. Pelvic muscle strength after childbirth. Obstet. Gynecol. 120, 1021–1028. ( 10.1097/aog.0b013e318265de39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XQ, Zhao Y, Pawlyk B, Damaser M, Li TS. 2006. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am. J. Pathol. 168, 519–528. ( 10.2353/ajpath.2006.050399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wieslander CK, Rahn DD, McIntire DD, Acevedo JF, Drewes PG, Yanagisawa H, Word RA. 2009. Quantification of pelvic organ prolapse in mice: vaginal protease activity precedes increased MOPQ scores in fibulin 5 knockout mice. Biol. Reprod. 80, 407–414. ( 10.1095/biolreprod.108.072900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. 2002. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am. J. Obstet. Gynecol. 187, 56–63. ( 10.1067/mob.2002.124843) [DOI] [PubMed] [Google Scholar]
- 12.Badiou W, Granier G, Bousquet PJ, Monrozies X, Mares P, de Tayrac R. 2008. Comparative histological analysis of anterior vaginal wall in women with pelvic organ prolapse or control subjects. A pilot study. Int. Urogynecol. J. 19, 723–729. ( 10.1007/s00192-007-0516-4) [DOI] [PubMed] [Google Scholar]
- 13.Northington GM, Basha M, Arya LA, Wein AJ, Chacko S. 2011. Contractile response of human anterior vaginal muscularis in women with and without pelvic organ prolapse. Reprod. Sci. 18, 296–303. ( 10.1177/1933719110392054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jallah Z, Liang R, Feola A, Barone W, Palcsey S, Abramowitch SD, Yoshimura N, Moalli P. 2016. The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG Int. J. Obstet. Gynaecol. 123, 1076–1085. ( 10.1111/1471-0528.13514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theofrastous JP, Swift SE. 1998. The clinical evaluation of pelvic floor dysfunction. Obstet. Gynecol. Clin. North Am. 25, 783–804. ( 10.1016/S0889-8545(05)70043-7) [DOI] [PubMed] [Google Scholar]
- 16.Dietz HP, Shek KL. 2008. The quantification of levator muscle resting tone by digital assessment. Int. Urogynecol. J. 19, 1489–1493. ( 10.1007/s00192-008-0682-z) [DOI] [PubMed] [Google Scholar]
- 17.Feola A, Moalli P, Alperin M, Duerr R, Gandley RE, Abramowitch S. 2011. Impact of pregnancy and vaginal delivery on the passive and active mechanics of the rat vagina. Ann. Biomed. Eng. 39, 549–558. ( 10.1007/s10439-010-0153-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoczylas LC, Jallah Z, Sugino Y, Stein SE, Feola A, Yoshimura N, Moalli P. 2013. Regional differences in rat vaginal smooth muscle contractility and morphology. Reprod. Sci. 20, 382–390. ( 10.1177/1933719112472733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basha M, Chang SH, Smolock EM, Moreland RS, Wein AJ, Chacko S. 2006. Regional differences in myosin heavy chain isoform expression and maximal shortening velocity of the rat vaginal wall smooth muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1076–R1084. ( 10.1152/ajpregu.00118.2006) [DOI] [PubMed] [Google Scholar]
- 20.Basha M, LaBelle EF, Northington GM, Wang TC, Wein AJ, Chacko S. 2009. Functional significance of muscarinic receptor expression within the proximal and distal rat vagina. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1486–R1493. ( 10.1152/ajpregu.90516.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntington A, Rizzuto E, Abramowitch S, Del Prete Z, De Vita R. 2018. Anisotropy of the passive and active rat vagina under biaxial loading. Ann. Biomed. Eng. 47, 272–281. ( 10.1007/s10439-018-02117-9) [DOI] [PubMed] [Google Scholar]
- 22.Fridez P, Makino A, Miyazaki H, Meister JJ, Hayashi K, Stergiopulos N. 2001. Short-term biomechanical adaptation of the rat carotid to acute hypertension: contribution of smooth muscle. Ann. Biomed. Eng. 29, 26–34. ( 10.1114/1.1342054) [DOI] [PubMed] [Google Scholar]
- 23.Baek S, Gleason RL, Rajagopal KR, Humphrey JD. 2007. Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput. Methods Appl. Mech. Eng. 196, 3070–3078. ( 10.1016/j.cma.2006.06.018) [DOI] [Google Scholar]
- 24.Jankowski RJ, Prantil RL, Fraser MO, Chancellor MB, de Groat WC, Huard J, Vorp DA. 2004. Development of an experimental system for the study of urethral biomechanical function. Am. J. Physiol. Renal Physiol. 286, F225–FF32. ( 10.1152/ajprenal.00126.2003) [DOI] [PubMed] [Google Scholar]
- 25.Makiyan Z. 2016. New theory of uterovaginal embryogenesis. Organogenesis 12, 33–41. ( 10.1080/15476278.2016.1145317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazloomdoost D, Westermann LB, Mutema G, Crisp CC, Kleeman SD, Pauls RN. 2017. Histologic anatomy of the anterior vagina and urethra. Female Pelvic Med. Reconstr. Surg. 23, 329–335. ( 10.1097/SPV.0000000000000387) [DOI] [PubMed] [Google Scholar]
- 27.Murtada SI, Ferruzzi J, Yanagisawa H, Humphrey JD. 2016. Reduced biaxial contractility in the descending thoracic aorta of fibulin-5 deficient mice. J. Biomech. Eng. Trans. ASME 138, 051008 ( 10.1115/1.4032938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferruzzi J, Murtada SI, Li GX, Jiao Y, Uman S, Ting MYL, Tellides G, Humphrey JD. et al. 2016. Pharmacologically improved contractility protects against aortic dissection in mice with disrupted transforming growth factor-beta signaling despite compromised extracellular matrix properties. Arterioscl. Thromb. Vasc. Biol. 36, 919–927. ( 10.1161/ATVBAHA.116.307436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanko LB, Mikkelsen EO, Frobert O, Bagger JP, Gregersen H. 1998. A new method for combined isometric and isobaric pharmacodynamic studies on porcine coronary arteries. Clin. Exp. Pharmacol. Physiol. 25, 919–927. ( 10.1111/j.1440-1681.1998.tb02344.x) [DOI] [PubMed] [Google Scholar]
- 30.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. 2007. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet. Gynecol. 109, 136–143. ( 10.1097/01.AOG.0000250472.96672.6c) [DOI] [PubMed] [Google Scholar]
- 31.Moalli PA, Debes KM, Meyn LA, Howden NS, Abramowitch SD. 2008. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am. J. Obstet. Gynecol. 199, 161.e1–161.e8. ( 10.1016/j.ajog.2008.01.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moalli PA, Howden NS, Lowder JL, Navarro JM, Debes KM, Abramowitch SD, Woo SL. 2005. A rat model to study the structural properties of the vagina and its supportive tissues. Am. J. Obstet. Gynecol. 192, 80–88. ( 10.1016/j.ajog.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 33.Robison KM, Conway CK, Desrosiers L, Knoepp LR, Miller KS. 2017. Biaxial mechanical assessment of the murine vaginal wall using extension-inflation testing. J. Biomech. Eng. Trans. ASME 139, 104504 ( 10.1115/1.4037559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akintunde A, Robison KM, Capone D, Desrosiers L, Knoepp LR, Miller KS. 2018. Effects of elastase digestion on the murine vaginal wall biaxial mechanical response. J. Biomech. Eng. 14, 021011 ( 10.1115/1.4042014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mei SS, Ye M, Gil L, Zhang JP, Zhang YP, Candiotti K, Takacs P. et al. 2013. The role of smooth muscle cells in the pathophysiology of pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 19, 254–259. ( 10.1097/SPV.0b013e31829ff74d) [DOI] [PubMed] [Google Scholar]
- 36.Gleason RL, Gray SP, Wilson E, Humphrey JD. 2004. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. J. Biomech. Eng. Trans. ASME 126, 787–795. ( 10.1115/1.1824130) [DOI] [PubMed] [Google Scholar]
- 37.Ferruzzi J, Bersi MR, Humphrey JD. 2013. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann. Biomed. Eng. 41, 1311–1330. ( 10.1007/s10439-013-0799-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin M, Le VP, Wagenseil JE. 2012. Mechanical testing of mouse carotid arteries: from newborn to adult. J. Vis. Exp. 60, 3733 ( 10.3791/3733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gravina FS, van Helden DF, Kerr KP, de Oliveira RB, Jobling P. 2014. Phasic contractions of the mouse vagina and cervix at different phases of the estrus cycle and during late pregnancy. PLoS ONE 9, e111307 ( 10.1371/journal.pone.0111307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baah-Dwomoh A, De Vita R. 2017. Effects of repeated biaxial loads on the creep properties of cardinal ligaments. J. Mech. Behav. Biomed. Mater. 74, 128–141. ( 10.1016/j.jmbbm.2017.05.038) [DOI] [PubMed] [Google Scholar]
- 41.Vanloon P, Klip W, Bradley E. 1977. Length–force and volume–pressure relationships of arteries. Biorheology 14, 181–201. ( 10.3233/BIR-1977-14405) [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, Rachev A, Shazly T. 2015. The biaxial active mechanical properties of the porcine primary renal artery. J. Mech. Behav. Biomed. Mater. 48, 28–37. ( 10.1016/j.jmbbm.2015.04.004) [DOI] [PubMed] [Google Scholar]
- 43.Ashoori F, Takai A, Tomita T. 1985. The response of non-pregnant rat myometrium to oxytocin in Ca-free solution. Br. J. Pharmacol. 84, 175–183. [PMC free article] [PubMed] [Google Scholar]
- 44.Caulk AW, Nepiyushchikh ZV, Shaw R, Dixon JB, Gleason RL. 2015. Quantification of the passive and active biaxial mechanical behaviour and microstructural organization of rat thoracic ducts. J. R. Soc. Interface 12, 20150280 ( 10.1098/rsif.2015.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panayi DC, Digesu GA, Tekkis P, Fernando R, Khullar V. 2010. Ultrasound measurement of vaginal wall thickness: a novel and reliable technique. Int. Urogynecol. J. 21, 1265–1270. ( 10.1007/s00192-010-1183-4) [DOI] [PubMed] [Google Scholar]
- 46.Humphrey JD. 2002. Cardiovascular solid mechanics: cells, tissues, and organs. Berlin, Germany: Springer. [Google Scholar]
- 47.Agianniotis A, Rachev A, Stergiopulos N. 2012. Active axial stress in mouse aorta. J. Biomech. 45, 1924–1927. ( 10.1016/j.jbiomech.2012.05.025) [DOI] [PubMed] [Google Scholar]
- 48.Zhou B, Prim DA, Romito EJ, McNamara LP, Spinale FG, Shazly T, Eberth JF. 2018. Contractile smooth muscle and active stress generation in porcine common carotids. J. Biomech. Eng. Trans. ASME 140, 014501 ( 10.1115/1.4037949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoka KV, et al. 2018. Effects of increased arterial stiffness on atherosclerotic plaque amounts. J. Biomech. Eng. Trans. ASME 140, 051007 ( 10.1115/1.4039175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruifrok AC, Johnston DA. 2001. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 23, 291–299. [PubMed] [Google Scholar]
- 51.Capone DJ, Clark GL, Bivona D, Ogola BO, Desrosiers L, Knoepp LR, Lindsey SH, Miller KS. 2019. Evaluating residual strain throughout the murine female reproductive system. J. Biomech. 82, 299–306. ( 10.1016/j.jbiomech.2018.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, Azadzoi KM. 1997. Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int. J. Impot. Res. 9, 27–37. ( 10.1038/sj.ijir.3900258) [DOI] [PubMed] [Google Scholar]
- 53.Berman JR. 2005. Physiology of female sexual function and dysfunction. Int. J. Impot. Res. 17, S44–S51. ( 10.1038/sj.ijir.3901428) [DOI] [PubMed] [Google Scholar]
- 54.Kelly BA, Chowienczyk P. 2002. An introduction to vascular biology. In An introduction to vascular biology (ed. Hunt BJ.), pp. 33–48. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 55.Geest JPV, Sacks MS, Vorp DA. 2006. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J. Biomech. 39, 1324–1334. ( 10.1016/j.jbiomech.2005.03.003) [DOI] [PubMed] [Google Scholar]
- 56.Fridez P, Makino A, Kakoi D, Miyazaki H, Meister JJ, Hayashi K, Stergiopulos N. 2002. Adaptation of conduit artery vascular smooth muscle tone to induced hypertension. Ann. Biomed. Eng. 30, 905–916. ( 10.1114/1.1507326) [DOI] [PubMed] [Google Scholar]
- 57.Humphrey JD, Dufresne ER, Schwartz MA. 2014. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812. ( 10.1038/nrm3896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daucher JA, Clark KA, Stolz DB, Meyn LA, Moalli PA. 2007. Adaptations of the rat vagina in pregnancy to accommodate delivery. Obstet. Gynecol. 109, 128–135. ( 10.1097/01.AOG.0000246798.78839.62) [DOI] [PubMed] [Google Scholar]
- 59.Wieslander CK, Marinis SI, Drewes PG, Keller PW, Acevedo JF, Word RA. 2008. Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biol. Reprod. 78, 521–528. ( 10.1095/biolreprod.107.063024) [DOI] [PubMed] [Google Scholar]
- 60.Ulrich D, et al. 2014. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PLoS ONE 9, e93172 ( 10.1371/journal.pone.0093172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kruger J, Hayward L, Nielsen P, Loiselle D, Kirton R. 2013. Design and development of a novel intra-vaginal pressure sensor. Int. Urogynecol. J. 24, 1715–1721. ( 10.1007/s00192-013-2097-8) [DOI] [PubMed] [Google Scholar]
- 62.Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyren O, Hammarstrom M. 2009. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet. Gynecol. 113, 1089–1097. ( 10.1097/AOG.0b013e3181a11a85) [DOI] [PubMed] [Google Scholar]
- 63.Rachev A, Hayashi K. 1999. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann. Biomed. Eng. 27, 459–468. ( 10.1114/1.191) [DOI] [PubMed] [Google Scholar]
- 64.Fan YH, Zhao JB, Liao DH, Gregersen H. 2005. The effect of digestion of collagen and elastin on histomorphometry and the zero-stress state in rat esophagus. Dig. Dis. Sci. 50, 1497–1505. ( 10.1007/s10620-005-2868-2) [DOI] [PubMed] [Google Scholar]
- 65.Gundiah N, Babu AR, Pruitt LA. 2013. Effects of elastase and collagenase on the nonlinearity and anisotropy of porcine aorta. Physiol. Meas. 34, 1657–1673. ( 10.1088/0967-3334/34/12/1657) [DOI] [PubMed] [Google Scholar]
- 66.Pichamuthu JE, Phillippi JA, Cleary DA, Chew DW, Hempel J, Vorp DA, Gleason TG. 2013. Differential tensile strength and collagen composition in ascending aortic aneurysms by aortic valve phenotype. Ann. Thorac. Surg. 96, 2147–2154. ( 10.1016/j.athoracsur.2013.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, Liu Y, Slipchenko MN, Zhao XF, Cheng JX, Kassab GS. 2011. The layered structure of coronary adventitia under mechanical load. Biophys. J. 101, 2555–2562. ( 10.1016/j.bpj.2011.10.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu X, Pandit A, Kassab GS. 2004. Biaxial incremental homeostatic elastic moduli of coronary artery: two-layer model. Am. J. Physiol. Heart Circul. Physiol. 287, H1663–H1669. ( 10.1152/ajpheart.00226.2004) [DOI] [PubMed] [Google Scholar]
- 69.Chen H, Slipchenko MN, Liu Y, Zhao XF, Cheng JX, Lanir Y, Kassab GS. et al. 2013. Biaxial deformation of collagen and elastin fibers in coronary adventitia. J. Appl. Physiol. 115, 1683–1693. ( 10.1152/japplphysiol.00601.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beamish JA, He P, Kottke-Marchant K, Marchant RE. 2010. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng. Part B-Rev. 16, 467–491. ( 10.1089/ten.teb.2009.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rensen SSM, Doevendans P, van Eys G. 2007. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart J. 15, 100–108. ( 10.1007/BF03085963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubod C, Boukerrou M, Brieu M, Dubois P, Cosson M. 2007. Biomechanical properties of vaginal tissue. Part 1: new experimental protocol. J. Urol. 178, 320–325. ( 10.1016/j.juro.2007.03.040) [DOI] [PubMed] [Google Scholar]
- 73.Feola A, Abramowitch S, Jones K, Stein S, Moalli P. 2010. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am. J. Obstet. Gynecol. 203, 8 ( 10.1016/j.ajog.2010.06.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pena E. et al. 2011. Mechanical characterization of the softening behavior of human vaginal tissue. J. Mech. Behav. Biomed. Mater. 4, 275–283. ( 10.1016/j.jmbbm.2010.10.006) [DOI] [PubMed] [Google Scholar]
- 75.Martins P, Silva AL, da Fonseca A, Santos A, Santos L, Mascarenhas T, Jorge RM, Ferreira AJ. 2013. Biomechanical properties of vaginal tissue in women with pelvic organ prolapse. Gynecol. Obstet. Invest. 75, 85–92. ( 10.1159/000343230) [DOI] [PubMed] [Google Scholar]
- 76.Ulrich D, Edwards SL, Letouzey V, Su K, White JF, Rosamilia A, Gargett CE, Werkmeister JA. 2014. Regional variation in tissue composition and biomechanical properties of postmenopausal ovine and human vagina. PLoS ONE 9, e104972 ( 10.1371/journal.pone.0104972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong RQ, Xia ZJ. 2019. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 234, 185–189. ( 10.1016/j.ejogrb.2019.01.012) [DOI] [PubMed] [Google Scholar]
- 78.Karam JA, Vazquez DV, Lin VK, Zimmern PE. 2007. Elastin expression and elastic fibre width in the anterior vaginal wall of postmenopausal women with and without prolapse. BJU Int. 100, 346–350. ( 10.1111/j.1464-410X.2007.06998.x) [DOI] [PubMed] [Google Scholar]
- 79.Clark GL, Pokutta-Paskaleva AP, Lawrence DJ, Lindsey SH, Desrosiers L, Knoepp LR, Bayer CL, Gleason RL Jr, Miller KS. 2019. Data from: Smooth muscle regional contribution to vaginal wall function Dryad Digital Repository. 100, 346–350. ( 10.5061/dryad.98bb788) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository [79].