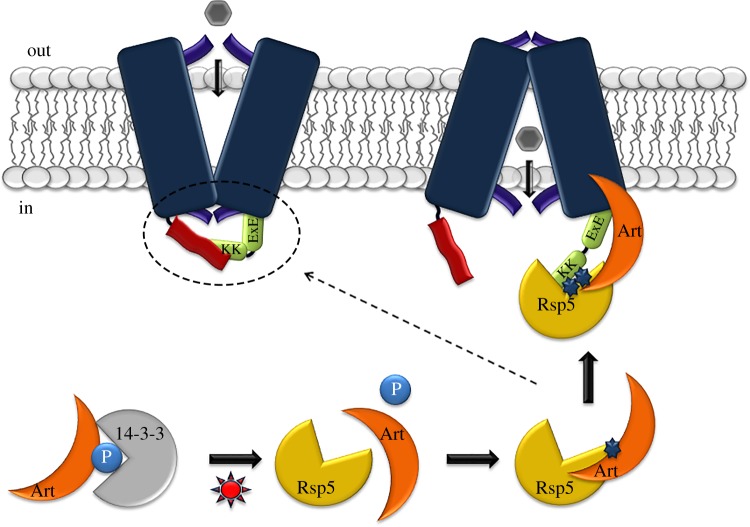
Figure 2.
Generalized model of transporter endocytosis highlighting the crucial role of N- and C-tails, based mostly on data concerning the FurE purine transporter, but also integrating critical findings from studies with Gap1 and Jen1 transporters (see text). The figure shows that in the outward-facing conformation the N- and C-tails are in close contact with each other and with other cytoplasmic domains of the transporter (e.g. the inner gate shown in purple), while in the inward-facing conformation the N- and C-tails separate to become more relaxed to recruit cytoplasmic effectors, such as those leading to ubiquitination and endocytosis. The figure includes a HECT-type ubiquitin ligase (Rsp5), its α-arrestin adaptor (Art) and a 14-3-3 protein that inhibits Art association with Rsp5 via phosphorylation. Upon a signal eliciting endocytosis (depicted by a blue-red star), Art is de-phosphorylated, acquires high affinity for Rsp5, which ubiquitylates Art, and the Art–Rsp5 complex is recruited to transporter tails. The relaxed topology of the cytosolic tails in the inward conformation permits more efficient recruitment of the Art–Rsp5 complex than the ‘hidden’ tails in the outward-facing conformation. The specific lysine residues (KK) and acidic motifs (e.g. EXE) necessary for ubiquitination are located either in the C-terminus (as in the figure) or in the N-terminus, but the interaction of termini is crucial for accessing these elements, so that both termini are critical for endocytosis.