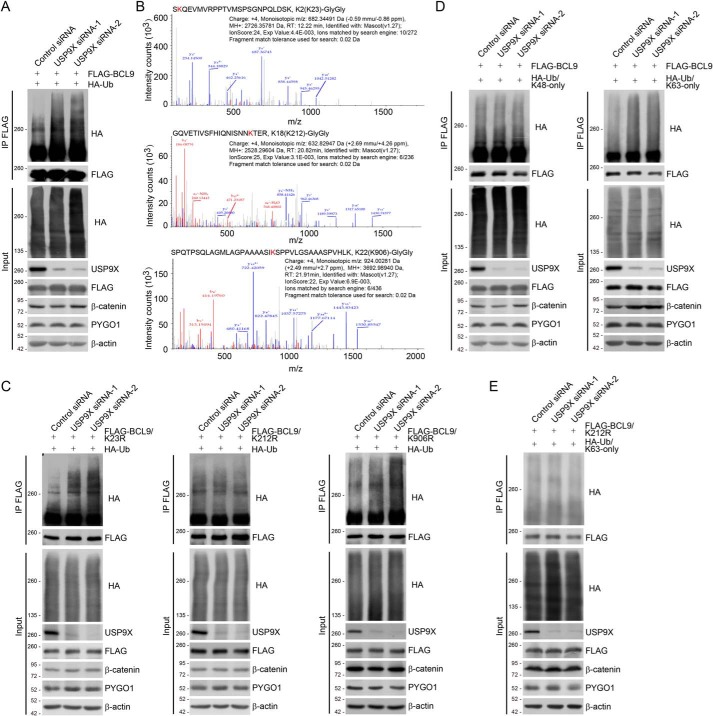
Figure 2.
USP9X deubiquitinates BCL9. A, HeLa cells with Dox-inducible expression of stably integrated FLAG–BCL9 were co-transfected with control siRNA or USP9X siRNAs together with HA–Ub as indicated. Cellular extracts were prepared for co-immunoprecipitation assays with anti-FLAG followed by IB with anti-HA. B, mass spectrometry analysis of BCL9 ubiquitin conjugation sites. HeLa cells stably expressing FLAG–BCL9 were co-transfected with HA–Ub. Cellular extracts were collected and sequentially purified with anti-FLAG affinity gel and HA affinity gel to enrich HA–Ub-conjugated BCL9. After trypsinization, the retrieved peptides were subjected to MS analysis. Fragmentation spectrums and parameters of the identified BCL9 peptides with the di-glycine remnants are shown. C, HeLa cells with Dox-inducible expression of stably integrated FLAG–BCL9/K23R, FLAG–BCL9/K212R, or FLAG–BCL9/K906R were co-transfected with control siRNA or USP9X siRNAs together with HA–Ub as indicated. Cellular extracts were prepared for co-immunoprecipitation assays with anti-FLAG followed by IB with anti-HA. D, HeLa cells with Dox-inducible expression of stably integrated FLAG–BCL9 were co-transfected with control siRNA or USP9X siRNAs together with HA–Ub/Lys-48–only or HA–Ub/Lys-63–only as indicated. Cellular extracts were prepared for co-immunoprecipitation assays with anti-FLAG followed by IB with anti-HA. E, HeLa cells with Dox-inducible expression of stably integrated FLAG–BCL9/K212R were co-transfected with control siRNA or USP9X siRNAs together with HA–Ub/Lys-63–only as indicated. Cellular extracts were prepared for co-immunoprecipitation assays with anti-FLAG followed by IB with anti-HA.