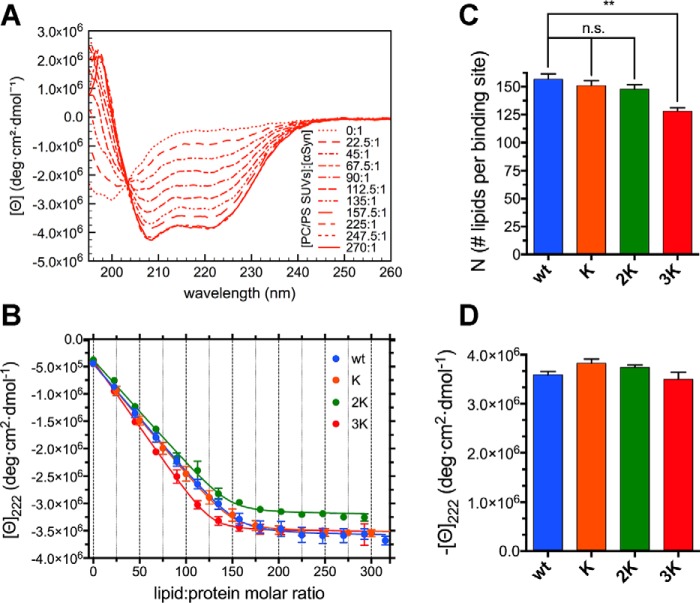
Figure 4.
Glu-to-Lys mutations result in mildly increased αSyn binding to curved charged membranes. A, representative far-UV CD spectra from a titration of 10 μm WT αSyn (in 10 mm NH4Ac (pH 7.4)) with 6 mm 70:30 POPC:DOPS SUVs, measured at 25 °C. There is a clear transition from the (almost completely) unstructured lipid-unbound protein to the robustly helical endpoints of the titration (e.g. lipid:protein molar ratio 270:1), reflecting the binding and folding of αSyn. B, titration curves obtained from raw CD data after extracting the molar ellipticity at 222 nm and graphing it against the lipid:protein molar ratio at each point of the titration. Datapoints are shown along with their standard errors (from n = 3 independent titrations) and their best fit with an N independent binding sites model (see “Experimental procedures”). C, histogram of the N values (along with their standard errors, n = 3) obtained from the best fit of the titration curves with an N-independent binding sites model. Decreasing Ns indicate increased binding (or avidity, see “Results”). D, histogram of the 222-nm plateau molar ellipticity values (along with their standard errors, n = 3) obtained from the end-point CD spectra of each mutant after renormalization using the WT αSyn isodichroic point. n.s., p > 0.05; **, p ≤ 0.01.