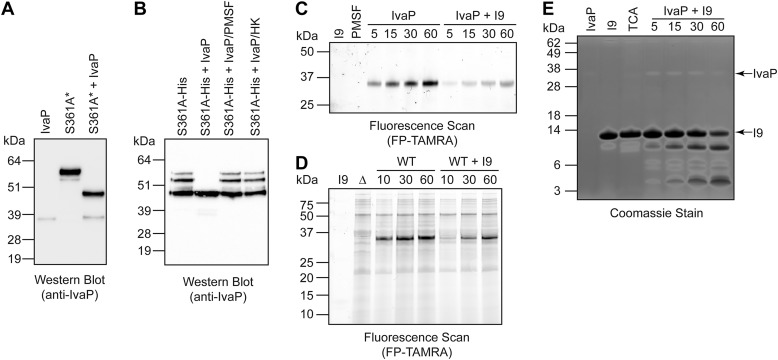
Figure 6.
The IvaP I9 domain is a temporary inhibitor and substrate of purified IvaP. Purified IvaP was incubated with stationary-phase culture supernatants from S361A* V. cholerae (A) or with purified IvaPS361A-His6 precursors (B) for 1 h at 37 °C prior to Western blot analysis. Cultures of S361A* were supplemented with ethanol to decrease proteolysis of IvaPS361A* as described under “Experimental procedures.” IvaP was pretreated with PMSF or heat-inactivated for 10 min at 95 °C (HK) for control experiments. C, purified IvaP was incubated with FP-TAMRA in the presence or absence of the purified I9 domain for 5–60 min at room temperature prior to in-gel fluorescence analysis. I9, propeptide alone treated with FP-TAMRA for 60 min. PMSF, IvaP alone pre-incubated with PMSF prior to treatment with FP-TAMRA for 60 min. As described under “Experimental procedures,” the molecular masses of the fluorescent protein standards are approximate. D, WT V. cholerae C6706 biofilm culture supernatants (WT) were incubated with FP-TAMRA in the presence or absence of the purified I9 domain for 10–60 min at room temperature prior to in-gel fluorescence analysis. I9, propeptide alone treated with FP-TAMRA for 60 min. Δ, biofilm culture supernatants from ΔivaP V. cholerae treated with FP-TAMRA for 60 min. As described under “Experimental procedures,” the molecular masses of the fluorescent protein standards are approximate. E, purified IvaP was incubated with the purified I9 domain for 5–60 min at 37 °C, followed by SDS-PAGE analysis and Coomassie staining. IvaP, IvaP alone incubated at 37 °C for 60 min. I9, propeptide alone incubated at 37 °C for 60 min. TCA, acid-inactivated IvaP incubated with the purified I9 domain for 60 min. These analyses were repeated three times with consistent results.