Abstract
Background
Human milk banking has been available in many countries for the last three decades. The milk provided from milk banking is predominantly term breast milk, but some milk banks provide preterm breast milk. There are a number of differences between donor term and donor preterm human milk.
Objectives
To determine the effect of banked donor preterm milk compared with banked donor term milk regarding growth and developmental outcomes in very low birth weight infants (infants weighing less than 1500 grams).
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 7), MEDLINE via PubMed (1966 to 23 October 2018), Embase (1980 to 23 October 2018), and CINAHL (1982 to 23 October 2018). We also searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised trials comparing banked donor preterm milk with banked donor term milk regarding growth and developmental outcomes in very low birth weight infants
Data collection and analysis
We planned to perform assessment of methodology regarding blinding of randomisation, intervention and outcome measurements as well as completeness of follow‐up. We planned to evaluate treatment effect using a fixed‐effect model using relative risk (RR), relative risk reduction, risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) for categorical data; and using mean, standard deviation and weighted mean difference (WMD) for continuous data. We planned to use the GRADE approach to assess the quality of evidence.
Main results
No studies met the inclusion criteria.
Authors' conclusions
We found no evidence to support or refute the effect of banked donor preterm milk compared to banked term milk regarding growth and developmental outcomes in very low birth weight infants.
Plain language summary
Banked preterm versus banked term human milk to promote growth and development in very low birth weight infants
Review question When mother's own milk is not available or is insufficient, does feeding banked donor preterm milk compared to banked donor term milk result in improved growth and neurodevelopmental outcome in very low birthweight infants?
Background Donor‐expressed milk processed by human milk banks has been used to provide preterm infants with breast milk when there are circumstances that preclude the use of mother's own milk. Preterm milk differs significantly from term breast milk. The processes involved in providing donor milk, including freezing, thawing and pasteurisation has adverse effects on nutritional and non‐nutritional aspects of donor milk. Donor milk is expensive. We wished to determine whether the benefits of preterm donor milk were superior to term donor milk either as a sole diet, or as a supplement to mother's own milk.
Study characteristics We were unable to identify any studies that compared donor preterm milk with donor term milk to promote growth and development in very low birth weight infants. Evidence is up to date as of October 2018.
Key results We were unable to conclude that donor preterm milk was superior to term donor milk as there is no evidence to support or refute this question. However the lack of studies identified in the original review and now updated in this review means that it is extremely unlikely that any such study will be performed.
Quality of evidence There were no studies to make an assessment of quality of evidence.
Background
Description of the condition
Mother's own milk is the milk of choice when feeding the preterm very low birth weight infant. Human milk provides a variety of benefits compared to formula. In preterm infants, reported benefits include faster gastric emptying (Cavell 1981; Ewer 1994), faster attainment of full enteral feeding (Uraizee 1989; Lucas 1990), enhanced stimulation of gastrointestinal motility and improved intestinal growth and maturation (Sheard 1988; Groer 1996). Breast milk is associated with a reduction in the incidence of necrotising enterocolitis (NEC) and late‐onset sepsis (Narayanan 1984; Schanler 1999). Preterm infants fed human milk appear to have improved neurodevelopmental outcome compared with infants fed formula milk (Anderson 1999). This association has been supported in the extremely low birth weight (ELBW) infant (Vohr 2006). Neonates fed breast milk tend to have improved visual development with less retinopathy of prematurity (Hylander 1995). However, there are circumstances when mother's breast milk may not be available. These circumstances occur when mothers cannot provide their own milk due to illness, inability to produce breast milk or due to concerns regarding certain prescription medications.
Description of the intervention
Human milk banking has been available in many countries for the last three decades and has played an important role in neonatal care. There are a number of donor milk banks in North America, United Kingdom, Europe and Australia. Human milk is donated to the milk bank voluntarily. Donors are screened for HIV, hepatitis B and C, human T‐lymphotropic virus and syphilis (Gibbins 2013). There has been a significant growth in the number of milk banks internationally. The screening, processing and shipping of donor breast milk incur considerable costs. The cost per ounce of expressed milk supplied to institutions varies from country to country. In the USA the estimated cost is USD 3 per ounce; in the UK it is GBP 3 per ounce, including processing and shipping costs (Arnold 2002).
Donor‐expressed milk has been used to provide preterm infants with breast milk when circumstances otherwise preclude the use of mother's own milk. Exclusive feeding with donor breast milk has been shown to reduce the incidence of NEC when compared to formula (McGuire 2003; Boyd 2006), but growth was slower. This benefit was only seen when breast milk was the sole dietary source. Some studies using donor‐banked milk or formula as a supplement to mother's own milk did not find any significant differences in reduction of NEC (Lucas 1984; Schanler 2005). There appears to be no evidence supporting enhanced long‐term outcome in infants fed donor milk (Modi 2006; Quigley 2018).
How the intervention might work
The milk provided from milk banking is predominantly term breast milk (often produced later in lactation so it has a different nutrient content), although many breast milk banking services now also batch and provide donated preterm breast milk (Tully 2001; Wight 2001). There are a number of differences between term and preterm human milk (Gidrewicz 2014). The nutritional and non‐nutritional components of human milk differ as gestational age advances (Boyce 2016). The relative constituents of protein, fat and carbohydrate differ (Gross 1980; Butte 1984), as do the non‐nutritional components including variations in digestive hormones, growth factors, immunological factors, vitamins, minerals and trace elements (Schanler 1980; Saarela 2005). Gidrewicz highlighted a preterm milk protein content of 2.2 g/dL and a fat content of 2.6 g/dL compared to term milk protein content of 1.8 g/dL and a fat content of 2.2 g/dL in the first week of life (Gidrewicz 2014). Long chain polyunsaturated fatty acids (LCPUFA) found in both term and preterm milk may differ. LCPUFA play an important role in optimal brain development and retinal maturation (Genzel‐Boroviczeny 1997). Donor breast milk undergoes a number of different processes including freezing and pasteurisation. These processes alter the nutritional composition of the milk and may affect preterm donor milk in a different way to term donor milk. Pasteurisation affects nutritional components resulting in slightly slower growth of infants on donor breast milk compared to raw unpasteurized human milk (Stein 1986). Pasteurisation affects immunological factors resulting in lower levels of lactoferrin and IgG. It eliminates white blood cells and bacteria. Despite pasteurisation IgA, bifid growth factor and lysozyme remain intact (Ford 1977). Holder pasteurisation (62.5 °C for 30 minutes) seems to be superior to heat treatment at 56 °C for 30 minutes in terms of cytomegalovirus elimination (Evans 1978). Freezing will eliminate most viruses and does not appear to influence nutritional quality of the milk (Wight 2001). Freezing does reduce the concentration of lysozyme by up to 20%, and also destroys all white blood cells. Microwaving affects the milk in the same way as described with pasteurisation (Quan 1992). Other newer methods of pasteurisation may have different effects on donor milk composition.
Why it is important to do this review
The inherent nutritional and non‐nutritional components of preterm and term donor milk differ (Gidrewicz 2014). The effects of freezing and pasteurisation alter the composition of donor milk, and may alter preterm and term donor milk differently. Therefore, the effect of banked donor preterm milk compared to donor term milk in feeding the very low birthweight infant warrants further investigation.
Objectives
To determine the effect of banked donor preterm milk compared with banked donor term milk regarding growth and developmental outcomes in very low birth weight infants (infants weighing less than 1500 grams).
The following comparisons were planned.
Any banked donor preterm milk (with or without fortification) versus any banked donor term milk (with or without fortification)
Banked donor preterm milk (with or without fortification) versus banked donor term milk (with or without fortification) where both were used as sole enteral diet
Any banked donor preterm milk (with or without fortification) versus any banked donor term milk (with or without fortification) in the extremely low birth weight infant
Methods
Criteria for considering studies for this review
Types of studies
Trials using randomisation or quasi‐randomisation of patients were eligible for inclusion. Published or unpublished studies were eligible for inclusion. We would have included unpublished studies or studies published only as abstracts if assessment of study quality was possible and if other criteria for inclusion were fulfilled.
Types of participants
Very low birth weight infants (infants weighing less than 1500 grams) fed donor banked human milk. Infants receiving partial enteral feeding (formula or mother's own milk) at study entry were eligible.
Types of interventions
Use of banked donor preterm milk versus banked donor term milk with or without fortification fed either as a sole enteral diet or as a supplement to mother's own milk.
Types of outcome measures
Primary outcomes
Short‐term growth parameters: time to regain birth weight (days of life), weight gain (grams/day), length gain (centimetres/week), head growth (centimetres/week) at discharge;
Longer‐term growth parameters (following discharge from hospital): weight gain (grams/week), length gain (centimetres/week), head growth (centimetres/week) at four months' follow‐up;
Neurodevelopmental outcomes at term corrected age and at 18 to 24 months using validated assessment tools.
Secondary outcomes
Incidence of necrotising enterocolitis (defined as Bell's Stage 2 or greater);
Incidence of late onset sepsis;
Duration of total parenteral nutrition use (days);
Time to full enteral feeds (days);
Feeding intolerance defined as abdominal distension with large gastric residuals (> 50% of previous feed).
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal.
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 7 in the Cochrane Library), MEDLINE via PubMed (1966 to 23 October 2018), Embase (1980 to 23 October 2018) and CINAHL (1982 to 23 October 2018) using the search terms detailed in Appendix 1. We did not apply language restrictions. This search was an update of the searches run for the review first published in 2010 (see Appendix 2 for previous search details) (Dempsey 2010).
We searched clinical trial registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en; and the ISRCTN Registry) on 23 October 2018.
Searching other resources
Other searches included reference lists of all selected articles as well as review articles. We also searched unpublished, in press and in progress trials and abstracts from neonatal and paediatric meetings. This included the proceedings of the Pediatric Academic Society meetings (PAS electronic version from 2000 to 2018) and the European Society for Paediatric Research meetings (from 2000 to 2018). Trials reported only as abstracts were considered eligible if there was sufficient information available from the report or from contact with the authors.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
We applied machine learning using the Cochrane Classifier tool in the Cochrane Register of Studies (CRS) to assess, then remove, reports with the least (0% to 2%) probability of being randomised controlled trials and with the least (0% to 2%) probability of having infants in the population. Each review author independently searched for trials and selected studies for inclusion with comparison and resolution of any differences. We illustrate our findings in a flowchart (Figure 1).
1.
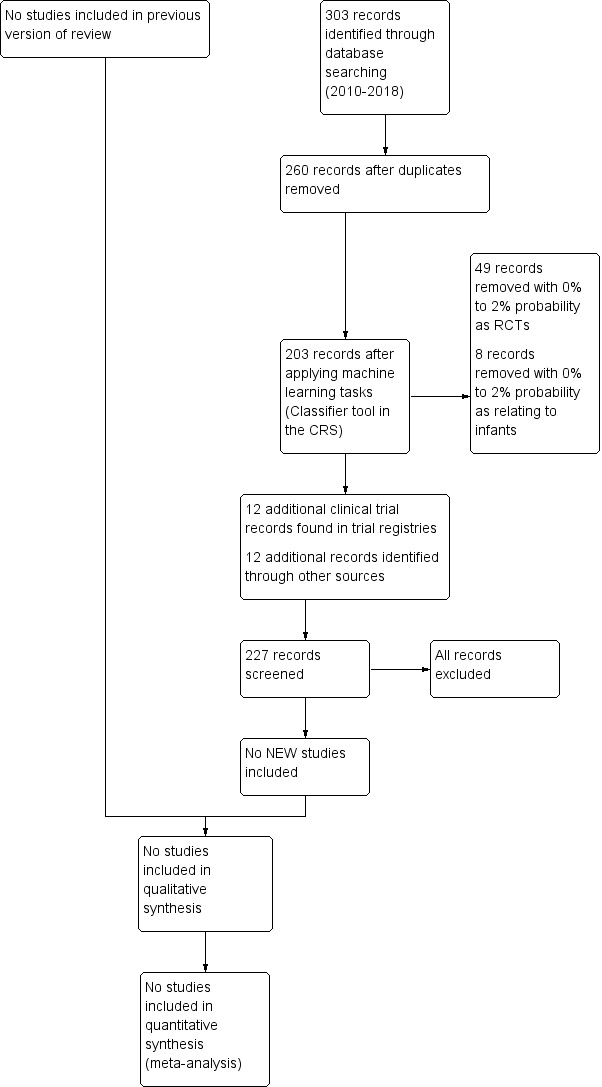
Study flow diagram: review update
Data extraction and management
If eligible trials had been identified, each review author planned to independently extract the data and compare results. We planned to use a data collection form to aid extraction of information on design, methodology, participants, intervention outcomes and treatment effects from included studies. We planned to resolve any disagreements through discussion.
Assessment of risk of bias in included studies
The two review authors planned to independently assess the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool, as described in Higgins 2017, for the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We planned to resolve any disagreements by discussion or by inviting a third assessor to arbitrate. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We planned to evaluate categorical data by calculating the relative risk (RR), relative risk reduction, risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). We intended to obtain mean and standard deviation for continuous data and perform analysis using the weighted mean difference (WMD). For each measure of effect, we planned to calculate the 95% confidence interval.
Unit of analysis issues
Our planned unit of analysis was the participating infant in individually randomised controlled trials.
Dealing with missing data
Where data were missing, we planned to deal with this by firstly contacting the relevant author. If we assumed that the data were missing at random, we planned to analyse the data without imputing any missing data.
Assessment of heterogeneity
We planned to examine heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If we detected statistical heterogeneity (I² > 50%) we planned to explore the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses.
Assessment of reporting biases
We planned to examine a funnel plot for asymmetry if there were more than 10 included trials.
Data synthesis
If we had identified multiple studies and judged that meta‐analysis was appropriate, we would have performed the analysis using Cochrane's Review Manager 5 software (Review Manager 2014). For estimates of typical relative risk and risk difference, we planned to use the Mantel‐Haenszel method. For measured quantities, we planned to use the inverse variance method. We intended to conduct all meta‐analyses using the fixed‐effect model.
Quality of evidence
We planned to use the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: growth, neurodevelopmental outcome, NEC and sepsis.
Review authors planned to independently assess the quality of the evidence for each of the outcomes above. We planned to consider evidence from randomised controlled trials as high quality but to downgrade the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We planned to use the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence(GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
The primary objective was to compare any banked preterm donor milk (with or without fortification) to any banked term donor milk (with or without fortification).
Subgroup comparisons of preterm donor milk with fortification to banked term donor milk with fortification and preterm donor milk without fortification to banked term donor milk without fortification were planned as following for each comparison.
Any banked donor preterm milk (with or without fortification) versus any banked donor term milk (with or without fortification)
The following subgroups were planned.
Banked preterm milk with fortification (using multi‐component breast milk fortifier) versus banked term milk with fortification (using multi‐component breast milk fortifier).
Banked preterm milk without fortification versus banked term milk without fortification.
Banked donor preterm milk (with or without fortification) versus banked donor term milk (with or without fortification) where both were used as sole enteral diet
The following subgroups were planned.
Banked donor preterm milk with fortification versus any banked donor term milk with fortification where both were used as sole enteral diet.
Any banked donor preterm milk without fortification versus any banked donor term milk without fortification where both were used as sole enteral diet.
Any banked donor preterm milk (with or without fortification) versus any banked donor term milk (with or without fortification) in the extremely low birth weightinfant
The following subgroups were planned.
Any banked donor preterm milk with fortification versus any banked donor term milk with fortification in the extremely low birth weight infant.
Any banked donor preterm milk without fortification versus any banked donor term milk without fortification in the extremely low birth weight infant.
Sensitivity analysis
There was no planned sensitivity analysis.
Results
Description of studies
Please see Figure 1 outlining the results of the search.
Results of the search
Please see Figure 1 outlining the results of the search.
Included studies
There were no new studies identified for inclusion in this review.
Excluded studies
See Characteristics of excluded studies table. A number of randomised clinical trials studies compared donor breast milk with formula (Gross 1983; Tyson 1983; Lucas 1990; Schanler 2005; Cristafolo 2013; Corpelejin 2016; O'Connor 2016); and some of these were the subject of a series of systematic reviews (McGuire 2003; Quigley 2007; Quigley 2014; Quigley 2018). None, however, compared donor preterm milk with donor term milk. One study compared pooled pasteurised breast milk with untreated mother's own milk but did not compare donor preterm milk with donor term milk (Stein 1986). Another study compared pooled pasteurised breast milk with untreated mother's own milk but again did not compare donor preterm versus term milk; it was also a before‐after study (Montjaux‐Régis 2011).
Risk of bias in included studies
No studies met the inclusion criteria.
Allocation
No studies met the inclusion criteria.
Blinding
No studies met the inclusion criteria.
Incomplete outcome data
No studies met the inclusion criteria.
Selective reporting
No studies met the inclusion criteria.
Other potential sources of bias
No studies met the inclusion criteria.
Effects of interventions
No studies met the inclusion criteria.
Discussion
We found no randomised controlled trials comparing banked preterm breast milk with banked term breast milk in the very low birth weight infant; therefore, this systematic review did not establish whether preterm donor milk conferred any health benefits compared to term donor milk fed solely, or as part of, the overall enteral diet of the very low birth weight infant. There have been a number of more recent studies comparing donor milk to formula for feeding the preterm infant and these have been the subject of a recent systematic review (Quigley 2018). This review highlighted reduction in NEC, but lower rates of weight gain, linear growth, and head growth in the donor milk group. There was no difference in all‐cause mortality or long‐term outcome in this systematic review. Each of these studies used pooled pasteurised breast milk, a combination of both preterm and (predominantly) term donor milk and so it is not possible to determine whether preterm donor milk had a greater influence on outcome compared with term donor milk. It is biologically plausible that preterm donor milk may confer additional benefits to the preterm infant compared to term donor milk because the nutritional components differ significantly. The non‐nutritional components also differ, including variations in digestive hormones, growth factors, immunological factors, vitamins, minerals and trace elements, all of which may contribute to improved well‐being in the preterm infant. The lack of included studies since the previous review and the lack of any current registered clinical trials highlight that it is unlikely that there would be any future randomised controlled trials comparing preterm donor milk with term donor milk, where each is the sole agent used. The most obvious reason is because of a very limited supply of donated preterm milk to milk banks. The vast majority of donors are from mothers who have delivered full‐term and have a large milk supply. Thus the limited supply of preterm donor milk, either because of limited donated volumes or limited numbers of breast milk banks that supply pooled preterm donor breast milk, means performing such studies would be difficult. Future randomised controlled trials could potentially be performed comparing preterm with term banked breast milk as an adjunct to mother's own milk or as an adjunct to formula feeding in the very low birth weight infant. However the same limitations would apply as highlighted above, which make it extremely unlikely that any such trial would be performed. It is more likely that future studies would focus on optimisation of the pooled undifferentiated donor milk content in the form of individualized fortification regimes.
Summary of main results
No included studies
Overall completeness and applicability of evidence
No included studies
Quality of the evidence
No included studies
Potential biases in the review process
No potential biases identified
Agreements and disagreements with other studies or reviews
This is an update of the previous review. This review again identified no new studies.
Authors' conclusions
Implications for practice.
In this most recent update, again we found no randomised controlled trials that compared banked preterm milk versus banked term milk to promote growth and development in very low birth weight infants.
Implications for research.
Although it is biologically plausible that banked preterm milk may be more suitable than banked term milk in feeding the preterm infant, it is unlikely that there would be any future randomised controlled trials comparing either milk source as a sole agent or an adjunct to mother's own milk or formula feeding. It is more likely that future studies would focus on optimisation of pooled undifferentiated donor milk in very low birth weight infants via different milk fortification methods.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2020 | Amended | Typo in Declarations of interest section corrected |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 4 June 2019 | New citation required but conclusions have not changed | No new studies were found. |
| 23 April 2019 | New search has been performed | Updated review of Dempsey 2010. |
Acknowledgements
The Methods section of this review is based on a standard template used by Cochrane Neonatal. We would like to sincerely thank Ms Colleen Ovelman for her support in performing this review.
Appendices
Appendix 1. Search strategy
PubMed:
(((Milk, Human[MeSH] OR breastmilk[TW] OR ((human[TW] OR breast*[TW] OR mother*[TW] OR maternal[TW] OR expressed[TW]) AND milk*[TW]) OR milk ejection[MeSH] OR Breast Milk Expression[MeSH])) AND (Milk Banks[MeSH] OR bank*[TW] OR milkbank*[TW] OR donor*[TW] OR (donor*[TW] AND milk*[TW]) OR (bank*[TW] AND milk*[TW])) AND ((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR "low birth weight"[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))) Filters: Publication date from 2010/01/01
Embase:
1. exp breast milk/ 2. exp milk ejection/ 3. exp breast milk expression/ 4. ((human or breast* or mother* or expressed or maternal) and milk*).mp. 5. breastmilk.mp. 6. 1 or 2 or 3 or 4 or 5 7. exp milk bank/ 8. (milkbank* or bank* or donor*).mp. 9. ((bank* and milk*) or (donor* and milk*)).mp. 10. 7 or 8 or 9 11. exp prematurity/ 12. exp infant/ 13. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 14. 11 or 12 or 13 15. (human not animal).mp. 16. (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. 17. 14 and 15 and 16 18. 6 and 10 and 17 19. limit 18 to yr="2010 ‐Current"
CINAHL:
(breastmilk OR ((human OR breast* OR mother* OR maternal OR expressed OR ejection) AND milk*)) AND (bank* OR milkbank* OR donor* OR (donor* AND milk*) OR (bank* AND milk*)) AND (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Limited to 2010‐18
CENTRAL:
1MESH DESCRIPTOR Milk, Human EXPLODE ALL AND CENTRAL:TARGET 2MESH DESCRIPTOR Milk Ejection EXPLODE ALL AND CENTRAL:TARGET 3MESH DESCRIPTOR Breast Milk Expression EXPLODE ALL AND CENTRAL:TARGET 4breastmilk OR ((human OR breast* OR mother* OR maternal OR expressed) AND milk*) AND CENTRAL:TARGET 5#1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET 6MESH DESCRIPTOR Milk Banks EXPLODE ALL AND CENTRAL:TARGET 7bank* OR milkbank* OR donor* OR (donor* AND milk*) OR (bank* AND milk*) AND CENTRAL:TARGET 8#7 OR #6 AND CENTRAL:TARGET 9infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 10#5 AND #8 AND #9 AND CENTRAL:TARGET 112010 TO 2018:YR AND CENTRAL:TARGET 12#10 AND #11
Clinical Trial Registries:
Clinicaltrials.gov Search terms: ((banked OR donor*) AND (milk OR breastmilk)) OR milkbank* | Child | First posted from 01/01/2010 to 07/10/2020 ISRCTN.com: Search terms: (infant OR neonate) AND (((banked OR donor*) AND (breastmilk OR milk)) OR milkbank*) WHO: Search terms: (infant OR neonate) AND (((banked OR donor*) AND (breastmilk OR milk)) OR milkbank*) ‐ limited to “Clinical trials in children” January 2010 to current
Appendix 2. Previous search details
For the review published in 2010, electronic searches included a search of the Cochrane Neonatal Group specialized register and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, January 2010). We identified relevant studies by searching the following: (1) computerised bibliographic databases: MEDLINE (1966 to February 2010), EMBASE (1988 to February 2010) and Web of Science (1975 to February 2010); (2) the Oxford Database of Perinatal Trials. The electronic search included the following keywords "donor expressed milk", "banked expressed milk" and MeSH search terms "Infant, Newborn" AND "Milk, Human" AND "Milk Banks". We limited trials to clinical trials where 'limits' option was available. We applied no language restrictions.
Appendix 3. 'Risk of bias' tool
We planned to use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we planned to seek information regarding the method of randomisation, blinding and reporting of all outcomes of all the infants enrolled in the trial. We planned to assess each criterion as being at a low, high, or unclear risk of bias. Two review authors planned to separately assess each study. We planned to resolve any disagreement by discussion. We planned to add this information to the table 'Characteristics of included studies'. We planned to evaluate the following issues and enter the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we planned to categorize the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we planned to categorize the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we planned to categorize the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was planned to be assessed separately for different outcomes or class of outcomes. We planned to categorize the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we planned to categorize the methods used to blind outcome assessment. Blinding was planned to be assessed separately for different outcomes or class of outcomes. We planned to categorize the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we planned to describe the completeness of data including attrition and exclusions from the analysis. We planned to note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We planned to categorize the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we planned to describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we planned to compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we planned to contact study authors to gain access to the study protocol. We planned to assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we planned to describe any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We planned to assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Corpelejin 2016 | This study compared donor milk to formula. |
| Cristafolo 2013 | This study compared donor milk to formula. |
| Gross 1983 | This study compared human milk with formula. |
| McGuire 2003 | This was a systematic review of donor breast milk compared to formula. |
| Montjaux‐Régis 2011 | This study compared pooled pasteurised breast milk with that of untreated mother's own milk and was a 'pre‐post' study. |
| O'Connor 2016 | This study compared donor milk to formula |
| Quigley 2007 | This was a systematic review of donor breast milk compared to formula. |
| Quigley 2014 | This was a systematic review of donor breast milk compared to formula. |
| Quigley 2018 | This was a systematic review of donor breast milk compared to formula. |
| Schanler 2005 | This study compared donor human milk versus preterm formula as an adjunct to mother's own milk. |
| Stein 1986 | This study compared pooled pasteurised breast milk with untreated mother's own milk. |
| Tyson 1983 | This trial compared pooled bank milk with enriched formula. |
Differences between protocol and review
We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol. We updated the search methods for the 2019 publication.
Contributions of authors
Dr. Dempsey conceived and designed the review. Both review authors performed separate data collection for the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
ED has no interest to declare.
JM has not interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
In order to maintain the utmost editorial independence for this Cochrane Review, William McGuire — outside the Cochrane Neonatal core editorial team and not receiving any financial remuneration from the grant — was the Sign‐off Editor for this review. Additionally, a Senior Editor from the Cochrane Children and Families Network, Robert Boyle, assessed and signed off this Cochrane Review.
Edited (no change to conclusions)
References
References to studies excluded from this review
Corpelejin 2016 {published data only}
- Corpelejin WE, De Waard M, Christmann V, VanGoudoever JB, Jansen-van der Weide MC, Kooi EM, et al. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: the Early Nutrition study randomized clinical trial. JAMA Pediatrics 2016;170(7):654–61. [DOI: 10.1001/jamapediatrics.2016.0183 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cristafolo 2013 {published data only}
- Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. Journal of Pediatrics 2013;163(6):1592–5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gross 1983 {published data only}
- Gross SJ. Growth and biochemical response of preterm infants fed human milk or modified infant formula. New England Journal of Medicine 1983;308(5):237-41. [DOI] [PubMed] [Google Scholar]
McGuire 2003 {published data only}
- McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(1):F11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montjaux‐Régis 2011 {published data only}
- Montjaux-Régis N, Cristini C, Arnaud C, Glorieux I, Vanpee M, Casper C. Improved growth of preterm infants receiving mother’s own raw milk compared with pasteurized donor milk. Acta Paediatrica 2011;100(12):1548–54. [DOI] [PubMed] [Google Scholar]
O'Connor 2016 {published data only}
- O’Connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, et al, GTA DoMINO Feeding Group. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low birth-weight infants at 18 months: a randomized clinical trial. JAMA 2016;316(18):1897–905. [DOI: 10.1001/jama.2016.16144] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quigley 2007 {published data only}
- Quigley M, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002971.pub2] [DOI] [PubMed] [Google Scholar]
Quigley 2014 {published data only}
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002971.pub3] [DOI] [PubMed] [Google Scholar]
Quigley 2018 {published data only}
- Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD002971.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schanler 2005 {published data only}
- Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics 2005;116(2):400-6. [DOI] [PubMed] [Google Scholar]
Stein 1986 {published data only}
- Stein H, Cohen D, Herman AA, Rissik J, Ellis U, Bolton K, et al. Pooled pasteurized breast milk and untreated own mother's milk in the feeding of very low birth weight babies: a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 1986;5(2):242-7. [PubMed] [Google Scholar]
Tyson 1983 {published data only}
- Tyson JE, Lasky RE, Mize CJ, Blair-Smith N, Whyte R, Beer AE. Growth, metabolic response, and development in very-low-birth-weight infants fed banked human milk or enriched formula. I. Neonatal findings. Journal of Pediatrics 1983;103(1):95-104. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1999
- Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta - analysis. American Journal of Clinical Nutrition 1999;70(4):525-35. [DOI] [PubMed] [Google Scholar]
Arnold 2002
- Arnold L. The cost effectiveness of using banked donor milk in the neonatal intensive care unit: prevention of necrotising enterocolitis. Journal of Human Lactation 2002;18(2):172-7. [DOI] [PubMed] [Google Scholar]
Boyce 2016
- Boyce G, Watson M, Lazidis G, Reeve S, Dods K, Simmer K, et al. Preterm human milk composition: a systematic literature review. British Journal of Nutrition 2016;116(6):1033-45. [DOI] [PubMed] [Google Scholar]
Boyd 2006
- Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: a systematic review and meta - analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006 Mar 23 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Butte 1984
- Butte NF, Garza C, Johnson CA. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Human Development 1984;9(2):153-62. [DOI] [PubMed] [Google Scholar]
Cavell 1981
- Cavell B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatrica Scandinavica 1981;70(5):639-41. [PubMed] [Google Scholar]
Evans 1978
- Evans TJ, Ryley HC, Neale LM, Dodge JA, Lewarne VM. Effect of storage and heat on antimicrobial proteins in human milk. Archives of Disease in Childhood 1978;53(3):239-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ewer 1994
- Ewer AK, Durbin GM, Morgan ME, Booth IW. Gastric emptying in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1994;71(1):F24-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 1977
- Ford JE, Law BA, Marshall VM, et al. Influence of heat treatment of human milk on some of its protective constituents. Journal of Pediatrics 1977;90(1):29-35. [DOI] [PubMed] [Google Scholar]
Genzel‐Boroviczeny 1997
- Genzel-Boroviczeny O, Wahle J, Koletzko B. Fatty acid composition of human milk during the 1st month after term and preterm delivery. European Journal of Pediatrics 1997;156(2):142-7. [DOI] [PubMed] [Google Scholar]
Gibbins 2013
- Gibbins S, Wong S, Unger S, O'Connor D. Donor human milk for preterm infants: Practice considerations. Journal of Neonatal Nursing 2013;19:175-81. [Google Scholar]
Gidrewicz 2014
- Gidrewicz D, Fenton T. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Paediatrics 2014;14:216. [DOI: 10.1186/1471-2431-14-216] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 07 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Groer 1996
- Groer M, Walker WA. What is the role of preterm breast milk supplementation in the host defenses of preterm infants? Science vs. fiction. Advances in Pediatrics 1996;43:335-58. [PubMed] [Google Scholar]
Gross 1980
- Gross SJ, David RJ, Bauman L, Tomarelli RM. Nutritional composition of milk produced by mothers delivering preterm. Journal of Pediatrics 1980;96(4):641-4. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hylander 1995
- Hylander MA, Stobino DM, Dhanireddy R. Human milk feedings and retinopathy of prematurity among very low birthweight infants. Journal of Perinatology 1996;16:236. [DOI] [PubMed] [Google Scholar]
Lucas 1984
- Lucas A, Gore SM, Cole TJ, Bamford MF, Dossetor JF, Barr I, et al. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Archives of Disease in Childhood 1984;59(8):722-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1990
- Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336(8730):1519-23. [DOI] [PubMed] [Google Scholar]
Modi 2006
- Modi N. Donor breast milk banking. BMJ (Clinical Research Ed.) 2006;333(7579):1133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanan 1984
- Narayanan I, Prakash K, Murthy NS, Gujral VV. Randomised controlled trial of effect of raw and holder pasteurised human milk and of formula supplements on incidence of neonatal infection. Lancet 1984;2(8412):1111-3. [DOI] [PubMed] [Google Scholar]
Quan 1992
- Quan R, Yang C, Rubenstein S, Lewiston NJ, Sunshine P, Stevenson DK, et al. Effects of microwave radiation on anti-infective factors in human milk. Pediatrics 1992;89(4 Pt 1):667-9. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saarela 2005
- Saarela T, Kokkonen J, Koivisto M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatrica 2005;94(9):1176-81. [DOI] [PubMed] [Google Scholar]
Schanler 1980
- Schanler RJ, Oh W. Composition of breast milk obtained from mothers of premature infants as compared to breast milk obtained from donors. Journal of Pediatrics 1980;96(4):679-81. [DOI] [PubMed] [Google Scholar]
Schanler 1999
- Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103(6 Pt 1):1150-7. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sheard 1988
- Sheard NF, Walker WA. The role of breast milk in the development of the gastrointestinal tract. Nutrition Reviews 1988;46(1):1-8. [DOI] [PubMed] [Google Scholar]
Tully 2001
- Tully DB, Jones F, Tully MR. Donor milk: what's in it and what's not. Journal of Human Lactation 2001;17(2):152-5. [DOI] [PubMed] [Google Scholar]
Uraizee 1989
- Uraizee F, Gross SJ. Improved feeding tolerance and reduced incidence of sepsis in sick very low birth weight (VLBW) infants fed maternal milk. Pediatric Research 1989;25:298A. [Google Scholar]
Vohr 2006
- Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, Poole WK, NICHD Neonatal Research Network. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome on the extremely low birthweight infant at 18 months of age. Pediatrics 2006;118(1):e115-23. [DOI] [PubMed] [Google Scholar]
Wight 2001
- Wight NE. Donor human milk for preterm infants. Journal of Perinatology 2001;21(4):249-54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dempsey 2010
- Dempsey E, Miletin J. Banked preterm versus banked term human milk to promote growth and development in very low birth weight infants. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007644] [DOI] [PubMed] [Google Scholar]


