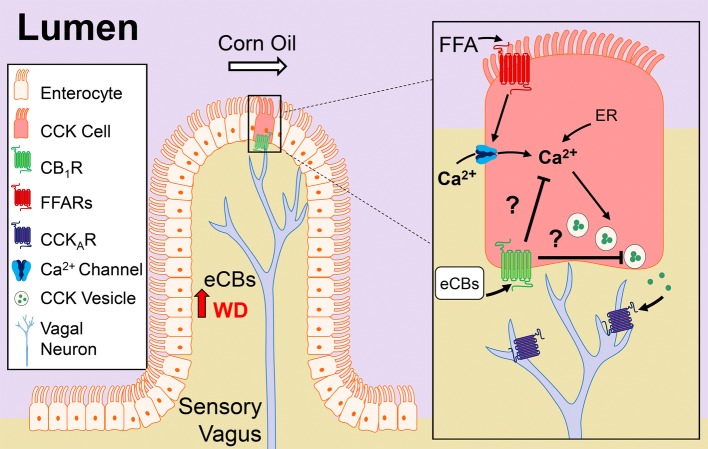
Figure 7.
Model of CB1R control of nutrient-induced CCK release. Our studies suggest that cannabinoid CB1Rs in the upper small-intestinal epithelium control nutrient-induced satiation signaling, and their signaling is increased in diet-induced obesity, which drives overeating by delaying satiation. The upper small-intestinal epithelium contains enteroendocrine I-cells, which are a subpopulation of enterocytes that secrete cholecystokinin (CCK) when nutrients – including dietary fats – enter the lumen (Rehfeld, 1998; Tanaka et al., 2008; Wang et al., 2011; Liou et al., 2011a; Brennan et al., 2012). Dietary fats (e.g., corn oil), in the form of triacylglycerols, are hydrolyzed in the lumen into mostly monoacylglycerols and free-fatty acids (FFAs) that are sensed by free-fatty acid receptors (FFARs) located on enteroendocrine cells in the small-intestinal epithelium. Activation of FFARs stimulates secretion of CCK by a mechanism that requires calcium (Ca2+) influx and/or intracellular (i.e., endoplasmic reticulum, ER) mobilization (McLaughlin et al., 1998; Hira et al., 2004; Tanaka et al., 2008). CCK activates CCKA receptors located on adjacent afferent sensory vagal fibers, which in turn, communicate with the brain and control meal size and satiation (Smith et al., 1981, 1985; Schwartz and Moran, 1994; Kaelberer et al., 2018). Consumption of a Western diet (WD) is associated with increased levels of the endocannabinoids (eCBs) and their activity at CB1Rs in the upper small-intestinal epithelium, which we propose inhibits CCK release and satiation signaling. The molecular mechanism(s) mediating CB1R control of CCK release is unknown, but may include inhibition of Ca2+-mediated CCK release. A future test of this hypothesis is warranted.