Abstract
Purpose
Scoliosis is a condition of abnormal growth resulting in 3D deformity of both the spine and thoracic cage. The aim of this study is to use chest radiographs of healthy children to define normal thoracic proportions so as to provide a useful normal reference range against which children with spinal deformity can be compared.
Methods
Three independent reviewers assessed posteroanterior and lateral chest radiographs of 184 normal children aged between two and 15 years. Duplicate assessments were undertaken by all three raters on 36 of these radiographs. We measured the T1 to T12 length, sternal length, chest depth at T6, chest width at T3, chest width at T6 and maximum chest width. Ratios of thoracic dimensions were calculated to define the normal proportions of the thorax. Inter- and intra-rater variance was estimated for all dimensions and dimension ratios.
Results
The intra-rater and inter-rater reliability was excellent with intra-class-correlation coefficients values > 80% and both intra- and inter-rater coefficients of variance < 9% for all parameters. All measured dimensions of the thorax and spine progressed linearly with respect to age. The mean proportions of T1 to 12 length of the sternal length, chest depth at T6, chest width at T3, chest width at T6 and maximum chest width were 0.5, 0.4, 0.7, 0.9 and 1.0, respectively.
Conclusion
It is possible to accurately and reproducibly measure the dimensions of the thoracic cage and spine on plain film radiology. The ratios of T1 to T12 length with respect to sternal length, chest depth at T6, chest width at T3, chest width at T6 and maximum chest remain constant with increasing age. Thoracic dimensions in children progress linearly with increasing age.
Level of Evidence
V
Keywords: spine, growth, thoracic, development, normal
Introduction
Instrumented spinal fusion to manage adolescent idiopathic scoliosis (AIS) is indicated when the deformity has reached a magnitude where further curve progression is inevitable even without ongoing thoracic growth.1 Spinal arthrodesis in younger children, with significant remaining growth leads to a disproportionally short thorax, a risk of progressive deformity requiring surgical intervention and can result in respiratory and metabolic compromise.2,3
A better understanding of thoracic insufficiency syndrome and the implications of early spinal arthrodesis has led to an increasing move towards growth preserving treatment strategies for younger children. A number of treatment modalities exist, ranging from casting, bracing, rib expansion devices and growing rods. Most of these strategies focus largely on increasing thoracic length (T1 to T12) rather than total thoracic size. There is a lack of available information on how thoracic proportions vary with age in healthy children.
The aim of this study is to use the plain film radiographs of healthy children to define normal thoracic proportions. This will provide a useful normal reference range against which children with spinal deformity can be compared. By gaining a better understanding of the differences in thoracic geometry between healthy children and those with spinal deformity, treatment strategies can be optimized.
Methods
We obtained ethical approval from Starship Children’s Hospital, Auckland, New Zealand. We searched the electronic hospital records and established a panel of all 6358 children who had a plain posteroanterior (PA) and lateral chest radiographs between the age of two and 15 years between July 2014 and January 2016. We then reviewed the medical records of each of the patients in this group and excluded any patient who demonstrated a pathological finding on the radiograph report, or who had a pre-existing underlying or subsequent medical diagnosis that might be expected to alter normal growth. Three trained reviewers (JK, TH, HU) independently evaluated these radiographs. Patients whose radiographs demonstrated a thoracic deformity or an anatomic variant (in having less than or more than 12 long ribs) or less than five osteogenic centres in the sternum were excluded from the study.4,5 Furthermore, all patients whose radiographs did not show the upper border of the first thoracic vertebrae and the lower border of the 12th thoracic vertebra or who demonstrated overinflation/underinflation were also excluded. A summary of exclusion criterion applied is given in Table 1.
Table 1.
Exclusion criterion
| Exclusion criterion |
|---|
| Less or more than 12 ribs bilaterally |
| Less or more than five osteogenic centres visible in the sternum |
| Not self-supported when radiograph was taken |
| Upper end plate of T1 not visible |
| Lower end plate of T12 not visible |
| Radiograph rotated to plate |
| Thoracic or spinal deformity visible on radiograph |
| Pathological finding on radiology report |
| Pre-existing or subsequent diagnosis of medical illness |
| Previous thoracic surgical procedure |
| Over/under inflation |
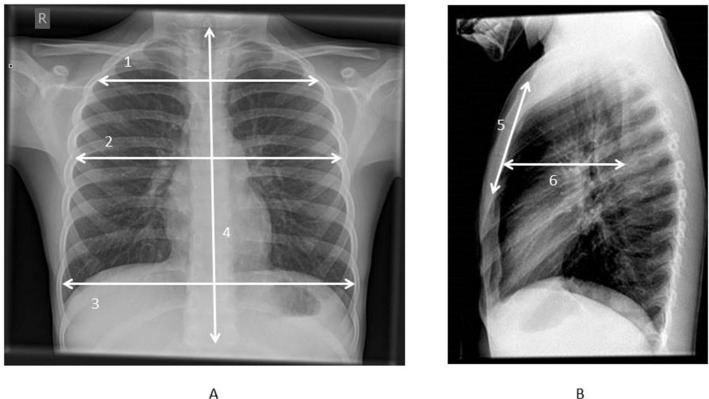
All radiographs were independently assessed on a picture archiving and communication system (PACS) workstation by each of the reviewers. The thoracic length was measured from the upper endplate of the T1 vertebral body to the lower endplate of the T12 vertebral body on the PA radiograph (Fig. 1a). The PA radiograph was also used to measure the maximum chest width from the medial cortex’s of horizontally opposite ribs (Fig. 1a). Chest widths at the midpoints of T3 and T6 vertebral bodies were also measured from the PA chest radiographs (Fig. 1a). The lateral radiograph was used to define the chest depth from the midpoint of the anterior border of T6 vertebral body horizontally to the posterior cortex of the sternum (Fig. 1b). The lateral radiograph was also used to measure the length of the sternum from the superior border of the manubrium to the inferior border of the most inferior osteogenic centre (Fig. 1b). Ratios of thoracic dimensions were calculated with respect to T1 to T12 length so as to describe the normal proportions of the thoracic cage.
Fig. 1.
(a) Parameters measured: 1) chest width at midpoint of T3, 2) chest width at midpoint of T6, 3) maximum chest width, 4) T1 to T12 length; (b) 5) sternal length, 6) chest depth at midpoint of T6.
To establish inter-rater reliability, all of the radiographs were reviewed independently by all three reviewers. A random group of 36 radiographs were reviewed a second time by the three reviewers who were blinded to their first measurements, to establish intra-reviewer reliability. The variability attributable to different raters and reassessments by the same rater were then estimated. This allowed the estimation of the inter-rater and intra-rater variance from which the coefficients of variation (CVs) and the intra-class-correlation coefficients (ICCs) could be calculated. Statistical analysis was conducted with the SPSS statistical package (version 23.0, SPSS Inc, Chicago, Illinois). Two-tailed values of p < 0.05 were considered to be significant.
Results
We reviewed 184 normal PA and lateral radiographs of children who meet the exclusion criterion given in Table 1. There were 70 female children and 114 males. A summary of the age distribution is given in Table 2.
Table 2.
Description of population by age
| Age | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Frequency | 10 | 10 | 10 | 15 | 17 | 22 | 9 | 20 | 17 | 9 | 10 | 10 | 10 | 15 |
| Percentage | 5.4 | 5.4 | 5.4 | 8.2 | 9.2 | 12.0 | 4.9 | 10.9 | 9.2 | 4.9 | 5.4 | 5.4 | 5.4 | 8.2 |
The results of intra and inter-rater variance calculation are shown in Table 3. The CVs for most raw measures and those adjusted by T1 to T12 length were generally very small (< 5%). Sternal length and chest depth were associated with more variation from between and within raters had CVs < 8%, further indicating that all dimensions can be measured precisely by individual and different raters. The ICCs indicating the percentage of the total variation are attributable to differences between participants. Most ICCs are > 80% (with five of the 11 measurements > 90%) indicating excellent reproducibility of the measurements. Sternal length and chest depth again show less precision with the sternal length ratio having the poorest ICC (40.2%), in part reflecting the difficulty in assessing the caudal end of the sternum in older more radiologically dense subjects.
Table 3.
Intra- and inter-observer reliability
| CV% raters | CV% intra-raters | ICC% | |
|---|---|---|---|
| T1 to T2 length | 0.00 | 1.70 | 99.10 |
| Maximum width | 0.20 | 0.98 | 99.60 |
| Width at T3 | 0.20 | 4.20 | 95.30 |
| Width at T6 | 0.44 | 2.74 | 97.30 |
| Sternal length | 7.05 | 8.05 | 81.40 |
| Chest depth at T6 | 4.56 | 6.25 | 84.00 |
| Maximum width/T1 to T12 length | 0.18 | 1.93 | 93.10 |
| Width at T3/T1 to T12 length | 0.90 | 4.30 | 82.30 |
| Width at T6/T1 to T12 length | 0.39 | 3.15 | 79.20 |
| Sternal length/T1 to T12 length | 6.88 | 7.90 | 39.00 |
| Chest depth at T6/T1 to T12 length | 4.56 | 6.33 | 72.90 |
CV, coefficient of variance; ICC, intra-class correlation coefficients
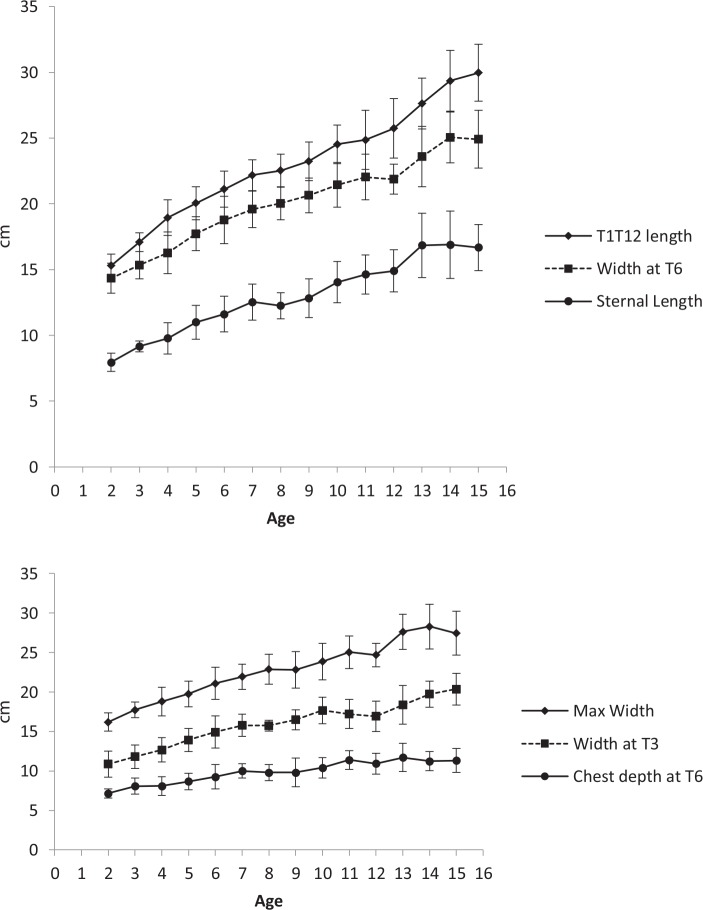
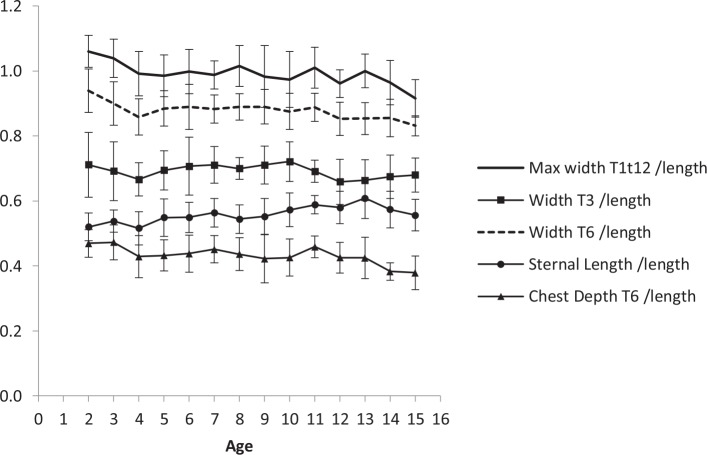
Linear patterns of the average thoracic dimensions were observed within the populations aged between two and 15 years (Fig. 2). When these dimensions are adjusted by T1 to T12 length they show a generally invariant pattern across the age groups (Fig. 3).
Fig. 2.
(a) The relationships between mean thoracic dimensions and chronological age; (b) the relationships between mean thoracic dimensions and chronological age.
Fig. 3.
Ratios of T1 to 12 length with respect to sternal length, chest depth at T6, chest width at T3, chest width at T6 and maximum chest width.
There are large statistically significant (all < 0.001) correlations amongst all thoracic dimensions (Table 4). The strong statistically significant correlations between age and thoracic dimensions indicate significant linear associations (Table 5).
Table 4.
Pearson’s correlation coefficients between thoracic dimensions (n = 184)
| T1 to T12 length | Maximum width | Width at T3 | Width at T6 | |
|---|---|---|---|---|
| Maximum width | 0.912 | |||
| Width at T3 | 0.875 | 0.843 | ||
| Width at T6 | 0.937 | 0.956 | 0.036 | |
| Sternal length | 0.914 | 0.905 | 0.819 | 0.8 |
All p < 0.001
Table 5.
Correlations between age and thoracic dimensions indicate significant linear associations
| Correlations | Age | |
|---|---|---|
| T1 to T12 length | r | 0.921* |
| p-value | 0.000 | |
| Maximum width | r | 0.846* |
| p-value | 0.000 | |
| Width at T3 | r | 0.821* |
| p-value | 0.000 | |
| Width at T6 | r | 0.878* |
| p-value | 0.000 | |
| Sternal length | r | 0.853* |
| p-value | 0.000 | |
| Chest depth at T6 | r | 0.666* |
| p-value | 0.000 | |
| Max width/T1 to T12 length | r | -0.339* |
| p-value | 0.000 | |
| Width T3/T1 to T12 length | r | -0.112 |
| p-value | 0.129 | |
| Width T6/T1 to T12 length | r | -0.326* |
| p-value | 0.000 | |
| Sternal length/T1 to T12 length | r | 0.301* |
| p-value | 0.000 | |
| Chest depth T6/T1 to T12 length | r | -0.338* |
| p-value | 0.000 |
Correlation is significant at the p<0.001 level (2-tailed)
Discussion
Our results show that it is possible to accurately and reliably measure thoracic dimensions from chest radiographs. We have also found that the T1 to T12 length is approximately equal to the maximum thoracic width in healthy children (ratio 1:1) and that the overall proportions of thoracic cage are relatively constant by the end of the second year of life (Fig. 3). Furthermore, we have determined that the dimensions of the thoracic cage progress in a linear fashion in normal children aged between two and 15 years (Fig. 2).
Dimeglio et al6,7 has made important contributions to our understanding of the growth of the thoracic cage and spine. They have done so by measuring the chest circumference, seated height and transverse and anteroposterior diameters of the thoracic cage using obstetric calipers. Dimeglio et al’s8 recent description of the external transverse and anteroposterior dimensions of the thoracic cage is in broad agreement with our results when accounting for differences in measurement method. We propose radiographic measurements as an alternative to Dimeglio’s technique because of the ease and feasibility of making our measurements on existing and/or historical radiographs readily available to spine surgeons in the outpatient clinic. Furthermore, by measuring radiographs acquired at consistent inspiration and in a standardized posture equivalent to that of a whole spine radiograph our findings are more reliable and transferable.
Roaf9 has published similar data on the variation of thoracic length. Their paper included a much smaller sample of 35 patients. The report did not state if they had excluded children with underlying pathologies. Importantly, Roaf9 contained no reference to the proportions of the thoracic cage relative to thoracic length.
Emans et al10 have studied the dimensions of the thoracic cage on CT scout views and axial reconstructions from CT scans. They found that thoracic length and depth are both strongly correlated with pelvic inlet width. They did not report the relationships between the dimensions of the thoracic cage or describe how the dimensions progressed with increasing age.
Our results will be clinically useful in a number of settings. Firstly, as a reference for those surgeons who perform hemiepiphysiodesis such as anterior tethering to correct spinal deformity.11-14 A better understanding of the age associated increase in T1 to T12 length based on routinely available radiological investigations will assist with both the timing and number of levels treated by these growth modulating techniques. Spine-anchored and rib-based growing constructs have been successfully used as a means of controlling thoracic deformity while preserving growth and maximizing pulmonary development.15,16 In this setting an appreciation of the constant ratio of maximum chest width with respect to thoracic length provided by this paper may be a useful guide as to the amount of length incrementally added to growing rod and rib-to-rib constructs so as to match normal proportions. Finally, in recent years there has been repopularization of de-rotational casts and orthosis to defer surgical treatment of children with infantile and early onset scoliosis.17-20 A reference of normal thoracic development and the concept of static thoracic proportions after the age of two years will be valuable to those who are measuring the effect on thoracic growth of deforming forces applied to the chest wall to manage spinal deformity in younger children.
We acknowledge that CT-based images could potentially allow more precise measurements to be taken. However, CT scanning has a number of limitations which would offset the gains in accuracy. Acquiring CT image sets of healthy children is difficult due to concerns relating to radiation exposure. CT data is captured in the supine position which alters the posture of the spine and thoracic cage which would change the proportions determined relative to a standing spine radiograph. A CT-based study would be less useful as plain film radiology is used to measure the progression of thoracic development in patients with early onset scoliosis in a normal outpatient setting. Finally, the use of plain film radiology to acquire proportions and dimensions will facilitate direct comparison between this normal group and those with thoracic deformity who have been imaged by standard radiographs in routine clinical practice. That said, a comparison between a plain radiograph study and a CT-based study examining lung volumes or formal pulmonary function-testing would be a valuable avenue for further research.
This paper represents an addition to the literature by measuring how thoracic proportions and dimensions in healthy children vary with age on routine plain film radiology which is the image modality most frequently used in clinical practice. We have established a database of the average proportions of the thoracic cage and spine in normal children between the ages of two and 15 years. In particular we have found that T1 to T12 length is approximately equal to maximum thoracic width in healthy children. These simple ratios form a reference against which children with spinal deformity can be easily compared using routine outpatient radiology.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
Ethical approval: Research involving human participants and/or animals was not performed. Institutional Review Board/Ethics committee approval was received.
Informed consent: Informed consent was not required.
ICMJE Conflict of interest statement
HC is a consultant to Orthopediatrics. All other authors have no conflict of interest to declare.
Author Contributions
JK: Study design, acquisition of data, drafting of manuscript.
TH: Acquisition of data.
HU: Acquisition of data.
CF: Study design, analysis of data, drafting of manuscript.
AH: Study design, analysis of data, drafting of manuscript.
PJK: Study design, drafting of manuscript.
HC: Study design, analysis of data, drafting of manuscript.
References
- 1.Weinstein SL, Dolan LA. The evidence base for the prognosis and treatment of adolescent idiopathic scoliosis: the 2015 Orthopaedic Research and Education Foundation Clinical Research Award. J Bone Joint Surg [Am] 2015;97:1899-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karol LA. Early definitive spinal fusion in young children: what we have learned. Clin Orthop Relat Res 2011;469:1323-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg CJ, Gillic I, Connaughton O, et al. . Respiratory function and cosmesis at maturity in infantile-onset scoliosis. Spine (Phila Pa 1976) 2003;28:2397-2406. [DOI] [PubMed] [Google Scholar]
- 4.Rush WJ, Donnelly LF, Brody AS, Anton CG, Poe SA. “Missing” sternal ossificationcenter: potential mimicker of disease in young children. Radiology 2002;224:120-123. [DOI] [PubMed] [Google Scholar]
- 5.O’Neal ML, Dwornik JJ, Ganey TM, Ogden JA. Postnatal development of the human sternum. J Pediatr Orthop 1998;18:398-405. [PubMed] [Google Scholar]
- 6.Dimeglio A. Growth in pediatric orthopaedics.. J Pediatr Orthop 2001;21:549-555. [PubMed] [Google Scholar]
- 7.Dimeglio ABF. Le rachis en croissance. Paris: Springer, 1990. [Google Scholar]
- 8.Dimeglio A, Bonnel FB, Canavese F. Normal growth of the spine and thorax Akbarnia BA, ed. The Growing Spine: Management of Spinal Disorders in Young Children. Berlin and Heidelberg: Springer, 2016:65-68. [Google Scholar]
- 9.Roaf R. Vertebral growth and its mechanical control. J Bone Joint Surg [Br] 1960;42-B:40-59. [DOI] [PubMed] [Google Scholar]
- 10.Emans JB, Ciarlo M, Callahan M, Zurakowski D. Prediction of thoracic dimensions and spine length based on individual pelvic dimensions in children and adolescents: an age-independent, individualized standard for evaluation of outcome in early onset spinal deformity. Spine (Phila Pa 1976) 2005;30:2824-2829. [DOI] [PubMed] [Google Scholar]
- 11.Samdani AF, Ames RJ, Kimball JS, et al. . Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976) 2014;39:1688-1693. [DOI] [PubMed] [Google Scholar]
- 12.Samdani AF, Ames RJ, Kimball JS, et al. . Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J 2015;24:1533-1539. [DOI] [PubMed] [Google Scholar]
- 13.Lavelle WF, Samdani AF, Cahill PJ, Betz RR. Clinical outcomes of nitinol staples for preventing curve progression in idiopathic scoliosis. J Pediatr Orthop 2011;31:S107-S113. [DOI] [PubMed] [Google Scholar]
- 14.Lavelle WF, Moldavsky M, Cai Y, Ordway NR, Bucklen BS. An initial biomechanical investigation of fusionless anterior tether constructs for controlled scoliosis correction. Spine J 2016;16:408-413. [DOI] [PubMed] [Google Scholar]
- 15.Emans JB, Caubet JF, Ordonez CL, Lee EY, Ciarlo M. The treatment of spine and chest wall deformities with fused ribs by expansion thoracostomy and insertion of vertical expandable prosthetic titanium rib: growth of thoracic spine and improvement of lung volumes. Spine (Phila Pa 1976) 2005;30:S58-S68. [DOI] [PubMed] [Google Scholar]
- 16.Akbarnia BA, Breakwell LM, Marks DS, et al. . Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine (Phila Pa 1976) 2008;33:984-990. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher ND, McClung A, Rathjen KE, et al. . Serial casting as a delay tactic in the treatment of moderate-to-severe early-onset scoliosis. J Pediatr Orthop 2012;32:664-671. [DOI] [PubMed] [Google Scholar]
- 18.Mehta MH. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J Bone Joint Surg [Br] 2005;87:1237-1247. [DOI] [PubMed] [Google Scholar]
- 19.Mehta MH. The conservative management of juvenile idiopathic scoliosis. Acta Orthop Belg 1992;58:91-97. [PubMed] [Google Scholar]
- 20.Baulesh DM, Huh J, Judkins T, et al. . The role of serial casting in early-onset scoliosis (EOS). J Pediatr Orthop 2012;32:658-663. [DOI] [PubMed] [Google Scholar]