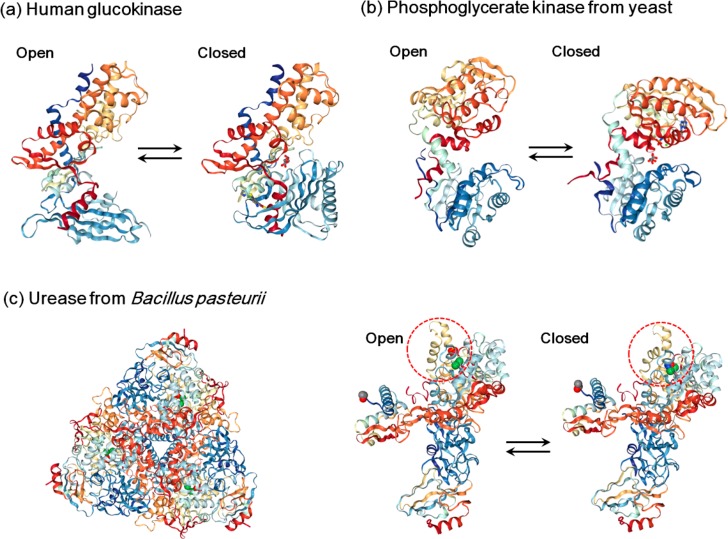
Figure 2.
Conformational changes of (a) human glucokinase, (b) phosphoglycerate kinase from yeast, and (c) urease from Bacillus pasteurii during the catalytic cycle. (a) For human glucokinase, the open conformation is shaped like a clamp with the active center at a groove (PDB entry 1 V4T). It closes upon glucose binding (PDB entry 1V4S). (b) Phosphoglycerate kinase from yeast possesses two lobes connected with a flexible hinge (open conformation, PDB entry 3PGK). The rotation of the hinge range brings the lobes closer, forming a closed conformation to initiate the reaction (closed conformation, PDB entry 1VPE). (c) Urease from Bacillus pasteurii is composed of a trimer of (αβγ)3 with a 3-fold symmetry (left, PDB entry 2UBP); each (αβγ) heteropolymeric assembly is a functional unit, and the α subunit holds the active site where two nickel ions (shown as green spheres) are bound. A helix–turn–helix motif acts as a flap (red-dashed circle) which can regulate the open (PDB entry 2UBP) and closed (PDB entry 3UBP) conformations during catalysis.