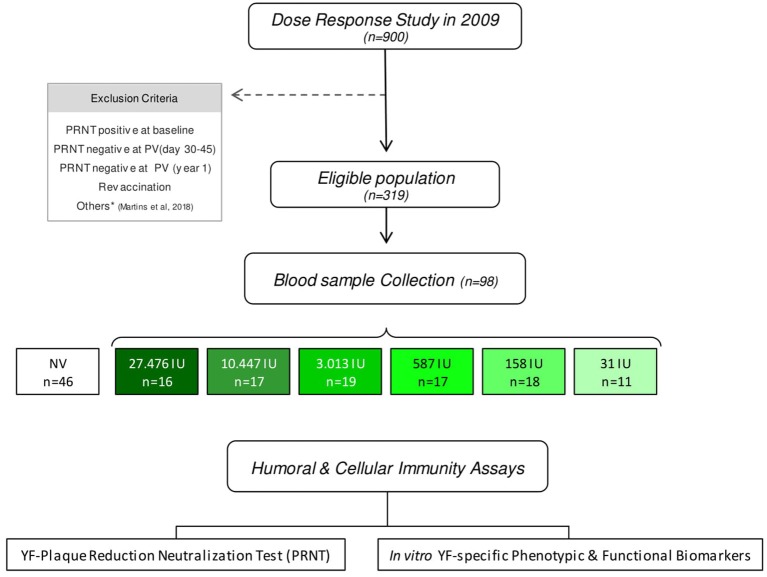
Figure 1.
Study design flowchart. The consort diagram summarizes the study steps. This is a 8-years follow-up study, of adult male army conscripts from Rio de Janeiro, average age of 19.4 years old, who had received the reference full dose and subdoses of 17DD-YF vaccine during the dose-response study in 2009 (12). From the eligible population (n = 319), only 98 volunteers agreed to provide two blood samples, one without anticoagulant for humoral analysis and an additional heparinized sample for cellular immunity assays. These volunteers were categorized into six groups, according to the dose of 17DD-YF vaccine administered in 2009: 27,476IU, considered the reference dose ( , n = 16); 10,447IU (
, n = 16); 10,447IU ( , n = 17); 3,013IU (
, n = 17); 3,013IU ( , n = 19); 587IU (
, n = 19); 587IU ( , n = 17); 158IU (
, n = 17); 158IU ( , n = 18), and 31IU (
, n = 18), and 31IU ( , n = 11). An additional group of non-vaccinated adult male army conscripts, referred as NV(day0), was included as a control (□, n = 46). Humoral and cellular immunity profile was determined for each volunteer using the YF-plaque reduction neutralization test (PRNT) and in vitro YF-specific phenotypic & functional biomarkers.
, n = 11). An additional group of non-vaccinated adult male army conscripts, referred as NV(day0), was included as a control (□, n = 46). Humoral and cellular immunity profile was determined for each volunteer using the YF-plaque reduction neutralization test (PRNT) and in vitro YF-specific phenotypic & functional biomarkers.