Abstract
Background
Lynch syndrome (LS) is a highly penetrant inherited cancer predisposition syndrome, characterized by autosomal dominant inheritance and germline mutations in DNA mismatch repair genes. Despite several genetic variations that have been identified in various populations, the penetrance is highly variable and the reasons for this have not been fully elucidated. This study investigates whether, besides pathogenic mutations, environment and low penetrance genetic risk factors may result in phenotype modification in a Tunisian LS family.
Patients and methods
A Tunisian family with strong colorectal cancer (CRC) history that fulfill the Amsterdam I criteria for the diagnosis of Lynch syndrome was proposed for oncogenetic counseling. The index case was a man, diagnosed at the age of 33 years with CRC. He has a monozygotic twin diagnosed at the age of 35 years with crohn disease. Forty-seven years-old was the onset age of his paternal uncle withCRC. An immunohistochemical (IHC) labeling for the four proteins (MLH1, MSH2, MSH6 and PMS2) of the MisMatchRepair (MMR) system was performed for the index case. A targeted sequencing of MSH2, MLH1 and a panel of 85 DNA repair genes was performed for the index case and for his unaffected father.
Results
The IHC results showed a loss of MSH2 but not MLH1, MSH6 and PMS2 proteins expression. Genomic DNA screening, by targeted DNA repair genes sequencing, revealed an MSH2 pathogenic mutation (c.1552C>T; p.Q518X), confirmed by Sanger sequencing. This mutation was suspected to be a causal mutation associated to the loss of MSH2 expression and it was found in first and second degree relatives. The index case has smoking and alcohol consumption habits. Moreover, he harbors extensive genetic variations in other DNA-repair genes not shared with his unaffected father.
Conclusion
In our investigated Tunisian family, we confirmed the LS by IHC, molecular and in silico investigations. We identified a novel pathogenic mutation described for the first time in Tunisia. These results come enriching the previously reported pathogenic mutations in LS families. Our study brings new arguments to the interpretation of MMR expression pattern and highlights new risk modifiers genes eventually implicated in CRC. Twins discordance reported in this work underscore that disease penetrance could be influenced by both genetic background and environmental factors.
Electronic supplementary material
The online version of this article (10.1186/s12967-019-1961-9) contains supplementary material, which is available to authorized users.
Keywords: CRC, Lynch syndrome I, MMR genes, DRGs, Tunisian family
Background
The surge in CRC incidence in young adults is particularly alarming. Among early onset-CRC, approximately 30% of patients are affected by tumors harboring mutations causing hereditary cancer predisposing syndromes, and 20% have familial CRC [1].
Lynch syndrome (LS) is considered as the most common hereditary CRC form [2]. It is an autosomal dominant syndrome subdivided into LS I, or site-specific colonic cancer, and LS II, or extracolonic cancer, with gastric, endometrial, biliary, pancreatic, and urinary tract carcinomas [3].
This syndrome is responsible of 2 to 6% of all CRC. It is known to increase the risk of other cancers in family members. The lifetime estimated risk for cancer ranges from 50 to 80% for CRC and from 40 to 60% for endometrial cancer [4]. Currently, in the context of lack of LS specific clinical symptoms, there is an important need to identify consistent molecular markers for early diagnosis and prognosis of this syndrome. In addition, it is for crucial importance to identify the mutational profile associated to LS in Tunisian population allowing us to implement an oncogenetic counseling based on genetic tests specific to this population. This will help in early detection of individuals and families at high risk of developing LS and will consequently reduce mortality and morbidity due to the disease. Indeed LS guidelines outline specific surveillance and monitoring protocols based on MMR genes mutation testing and MMR proteins expression profile [5, 6]. The MMR system is composed of four proteins working in pairs (dimers-MLH1/PMS2 and MSH2/MSH6). These dimmers migrate to the nucleus to bind to the DNA. The formation of the complex is crucial for the stability, migration and the function of the complex [7].
LS is characterized by point mutations and/or large rearrangements in DNA MMR genes [8] resulting in a loss of MMR complex function and microsatellite instability (MSI) [9, 10]. The high penetrance mutations confer a predisposition to CRC in hereditary syndromes, responsible for about 50–80% of risk to develop CRC [11, 12]. However, there is a large variability in LS penetrance that is essentially dependent on low penetrance mutations and environmental factors. In fact, recently, germline mutations in DNA-repair genes (DRGs) have been reported in sporadic CRC, but their contribution to CRC risk and susceptibility is still unclear. Germline mutations in DRGs previously known to be linked to other inherited diseases could be involved in familial CRC predisposition [13]. Moreover, both germline and somatic variants in the exonuclease domains of DNA polymerase ɛ (POLE) and polymerase (POLD1) have been reported to affect proofreading function and lead to an ultramutated phenotype [14]. Germline POLE variants can result in a LS phenotype and microsatellite instable CRCs. The exact effect of germline POLE/POLD1 variants remains however, unclear [14–17].
The identification of an inherited mutation plays a crucial role in identifying at risk individuals and families for LS that are proposed for oncogenetic counseling [18]. However, Neither MMR mutated gene nor mutation type are associated to the onset age or the cancer type [3]. Thus, it is for crucial importance to search for other DRGs that could be implicated in the increase of CRC risk in patients with strong familial history. Moreover, underlying genotype–phenotype correlation in LS provides significant insights for oncogenetic counseling of familial CRC.
So far, very few clinical studies and genetic reports conducted on patients with LS in Tunisia have been published [19, 20]. Moussa et al. [20] have identified pathogenic mutations in MMR genes in only 11/31 LS suspected Tunisian families. Given limitations to this previous Tunisian study, the CCR susceptibility genes list could be expended with new DNA repair genes. In this study our main goal is to identify germline mutations associated to LS in a CRC Tunisian family with monozygotic twins and to assess factors associated with increasing cancer risk.
Methods
Patients
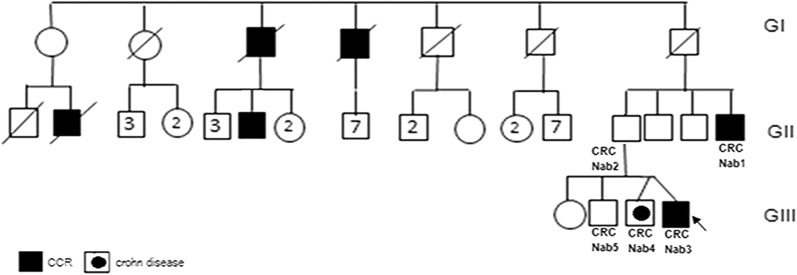
This study was conducted according to the declaration of Helsinki and to the approval of the Institutional reviewed board (IRB) of Institut Pasteur de Tunis. Five individuals, belonging to the same large Tunisian family, were investigated after written informed consent (Fig. 1). This family fulfill the Amsterdam I criteria for the diagnosis of Lynch syndrome. The index case was a man (CRCNab3), referred for a molecular diagnosis of LS to the Gastroenterology Department of Mohamed Tahar Maamouri Medical Hospital in Nabeul, Tunisia. He was diagnosed at 33-years-old with a well differentiated adenocarcinoma at the transverse colon (pT3 N1a of 6 cm × 6 cm × 2 cm) and treated with hemicolectomy. The index case has a monozygotic (MZ) twin diagnosed with crohn disease at the age of 35 (CRCNab4) and a brother who recently suffered from gastro-intestinal disconfort but considered as healthy (CRCNab5). Their father developed lymphoid hyperplasia at right colon without clinical significance (CRCNab2). Their paternal uncle (CRCNab1) was diagnosed with a sigmoidien well differentiated lieberkuhnien adenocarcinoma T3N0MX at 47-years-old treated with sigmoidectomy. The index case and his two brothers have smoking and/or alcohol consumption habits. The other investigated relatives have neither smoking nor alcohol consumption habits.
Fig. 1.
The familial pedigree of the HNPCC family. The index case family history included only colon cancer in second and third-degree relatives; it showed a tumor spectrum typical of LS I form
Immunohistochemical study
To assess the expression of MMR proteins, we performed immunohistochemical labeling on Formalin Fixed Paraffin Embedded (FFPE) sample from the CRC of (CRCNab3), using the four primary antibodies against MMR system [anti-MLH1 (ES05), anti-MSH2 (25D12), anti-MSH6 (PU29) anti-PMS2 (M0R4G)] Leica Biosystems. We used a sporadic CRC with proficient MMR (pMMR) status as control.
Targeted DNA repair genes panel conception (DRGs)
Library preparation for NGS was accomplished using the novel development of the HaloPlex assay that incorporates molecular barcodes for high-sensitivity sequencing as a custom design (HaloPlexHS). Using SureDesign (Agilent Technologies Inc.), probes were generated to cover the exons and 15 bp of the surrounding intronic sequences of a total of 87 candidate genes known to be involved in DNA repair disorders (the list of all analyzed genes is provided as Additional file 1). The size of the final target region was 251.689 kpb with 33828 amplicons and the mean coverage was 99.74% of the target region. Amplicon libraries were prepared, from genomic DNA of (CRCNab2) and (CRCNab3), using the HaloPlexHS PCR target enrichment system dedicated to Ion Torrent PGM according to the manufacturer’s recommendations. Massively parallel sequencing was performed on an Ion Torrent PGM (Thermo Fisher Scientific). Raw data generated by the PGM sequencer were analysed using the in-house VarAft software version 2.5, which is freely available online (http://varaft.eu/index.php). We prioritized rare functional variants (missense, nonsense, splice site variants, and indels) and excluded variants with a Minor Allele Frequency (MAF) > 0.01 in dbSNP137, and 138, in the Exome Variant Server (http://evs.gs.washington.edu/EVS/), 1000 Genomes Project (http://www.1000genomes.org/), or Exome Aggregation Consortium database (ExAC), Cambridge, MA (http://exac.broadinstitute.org). A number of online tools were used to predict the functional impact and pathogenicity of the identified variants such as MutationTaster (http://www.mutationtaster.org/), PredictProtein (https://www.predictprotein.org/), PolyPhen (http://genetics.bwh.harvard.edu/pph2/), Combined Annotation Dependent Depletion (CADD) (http://cadd.gs.washington.edu/), SIFT (http://sift.bii.a-star.edu.sg/) and UMD predictor (http://umd-predictor.eu/). Variants not previously reported in healthy controls and classified as pathogenic were evaluated for sequencing depth and visually inspected using the Integrative Genomic Viewer (IGV) before validation by Sanger Sequencing.
Sanger sequencing
PCR reactions were performed on genomic DNAs (gDNAs), following standard protocols, pursued by Sanger sequencing using an automated sequencer (ABI 3500; Applied Biosystems, Foster City, CA) using a cycle sequencing reaction kit (Big Dye Terminator kit, Applied Biosystems). Data were analyzed by BioEdit Sequence Alignment Editor Version 7.2.5. As the POLE/POLD1 genes were not included in the HaloPlex gene panel list, the Sanger sequencing was used to screen for the following hotspot pathogenic mutations: p.L424V located in exon 13 of POLE gene, and p.S478N located in exon 11 of POLD1 gene [15].
Results
Immunohistochemical pattern
IHC result for (CRCNab3) showed an MSH2 nuclear expression loss in tumor and in stromal cells, a cytoplasmic staining for MSH6 and PMS2 and an incomplete nuclear staining for MLH1. The sporadic CRC sample with proficient MMR (pMMR), showed a positive nuclear staining in tumor cells as well as in adjacent normal cells (Fig. 2) with all the proteins.
Fig. 2.
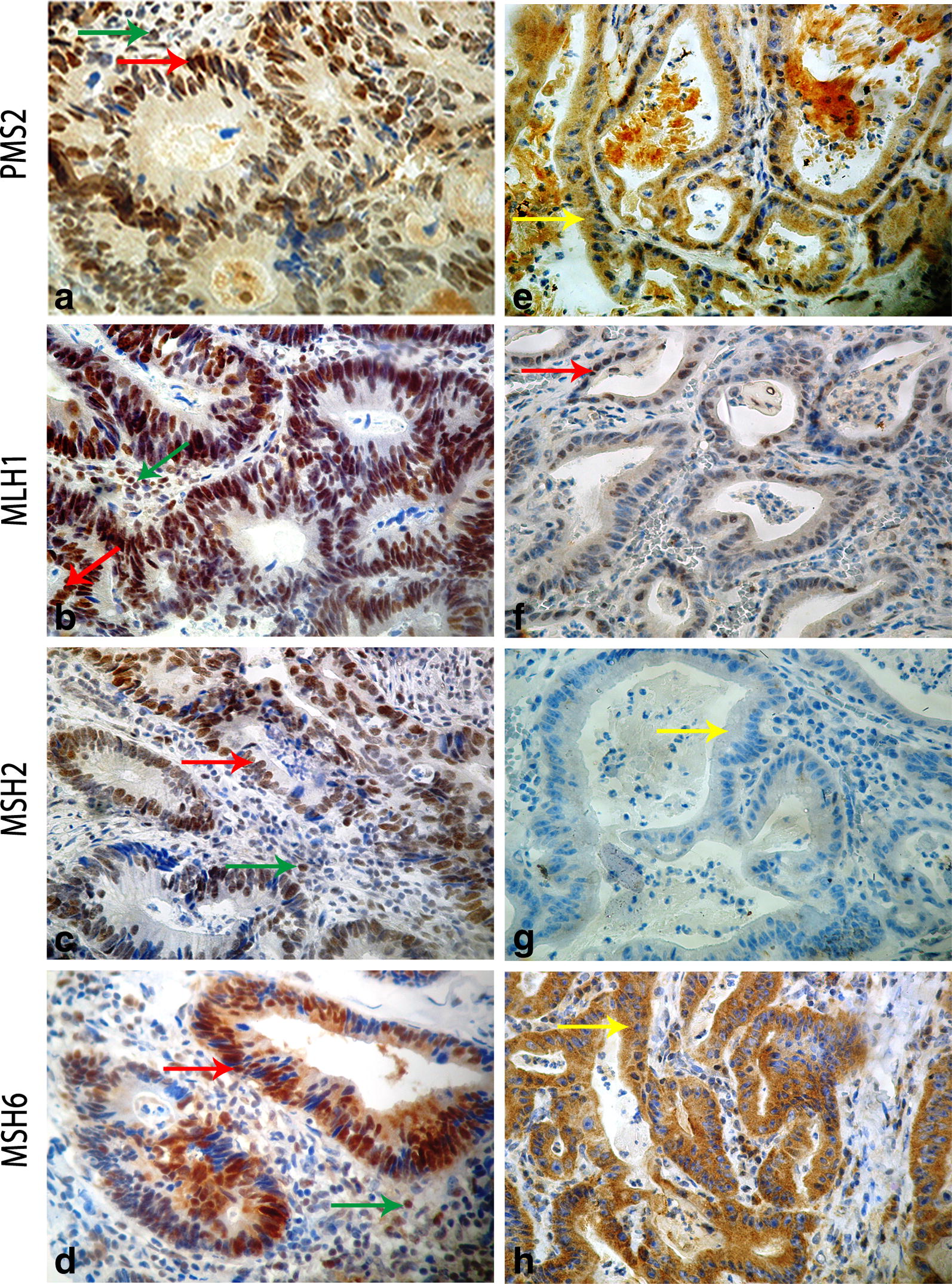
Representative MMR immunohistochemical expression patterns. Left panel: (a, b, c and d) are IHC pictures of a sporadic CRC sample with proficient MMR (pMMR). The pMMR sample staining shows strong nuclear expression in both tumor (red arrow) and adjacent stromal cells (internal control, green arrow) for the four used antibodies anti-PMS2 (a), anti-MLH1 (b), anti-MSH2 (c) and anti-MSH6 (d). Right panel: (e, f, g and h) are IHC pictures of the index case CRC with deficient MMR (dMMR). The dMMR proband staining showed: a loss of nuclear MSH2 expression in normal stromal and cancer cells (g) confirming the MMR deficiency, a nuclear incomplete staining for MLH1 (f) and a cytoplasmic staining for PMS2 (e) and MSH6 (h) (yellow arrow). Original magnifications (40x)
Pathogenic mutation detected by targeted DNA repair genes panel
Variants not previously reported in healthy controls and classified as pathogenic in ClinVar were evaluated for sequencing depth and visually inspected using the Integrative Genomic Viewer (IGV). After filtering strategies, described above, an MSH2 mutation (c.1552C>T; p.Q518X) was detected in both, the index case (CRCNab3) and his father (CRCNab2) and it was selected as candidate for Sanger validation, among the other identified variations.
Analysis of variants carried by the proband (CRCNab3) and absent in his father (CRCNab2)
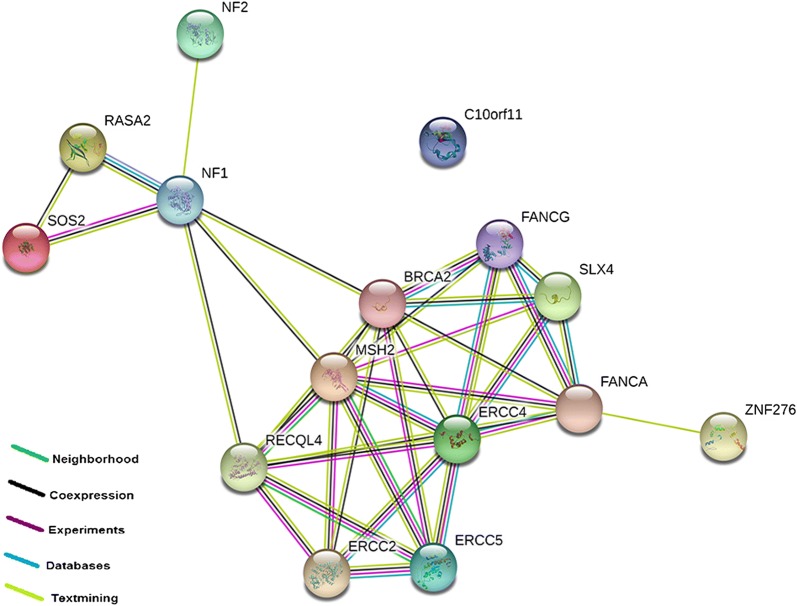
Eighty-seven variants on 60 genes have been detected in the proband (CRCNab3) not shared with his father (CRCNab2). After filtering steps (Table 1), fifteen non shared variants have been identified; 13 exonic variants, 1 splicing SNPs and 1 frameshift variant. We detected the following variations (Table 2): an SOS2 rare variant (rs532833599, MAF = 0.00019) and an LRMDA rare variant (rs763696041, MAF = 0.0005), ERCC2 (exon5:c.360+3G>T), ERCC4 (exon11: c.2422G>T: p.A808S), ERCC5 (exon 1: c.8T>G: p.V3G), BRCA2 exon 17 variation (c.7810C>A: p.L2604M), an exon 13 variation (c.2120G>T: p.C707F) in RECQL4, an exon 3 SLX4 variation (c.742G>T: p.E248X) and two NF1 variations in exon 25 (c.3259C>T: p.P1087S) and exon 43 (c.6623C>A:p. A2208D). Their respective protein interactions predictions are illustrated in Fig. 3 using String software Version 11.0.
Table 1.
Variants filtering results of targeted DNA repair genes panel for the index case
| Filtering conditions procedure | CRCNab3 | |
|---|---|---|
| Genes | Variants | |
| Total number | 87 | 268 |
| Variations present for the proband case and absent in his father | 60 | 87 |
| Missense, nonsense, splice-site or frameshift variants | 44 | 48 |
| MAF < 0.1% in the 1000 genome, ExAC | 35 | 37 |
| CADD score ≥ 15 | 14 | 15 |
MAF minor allele frequency, ExAC the Exome Aggregation Consortium, CADD combined annotation dependent depletion
Table 2.
Variants not shared between CRCNab3 and CRCNab2
| Chr | Gene | NM Number | Func.refgene | Start | AA change | Frequency (exac/1000 g) | dbsnp | ClinVar | UMD prediction | SIFT_pred |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | LRMDA | NM_001305581 | exon 6 | 78084234 | c.592A>G:p.S198G | 0.00005 (ExAC) | rs763696041 | NA | Pathogenic | Tolerated |
| 2 | MSH2 (other then the confirmed one) | NM_000251 | exon14 | 47705572 | c.2372C>A:p.A791D | NA | NA | NA | Pathogenic | Tolerated |
| 3 | RASA2 | NM_006506 | exon 1 | 141206014 | c.89A>T:p.D30V | NA | NA | NA | Pathogenic | Deleterious |
| 8 | RECQL4 | NM_004260 | exon 13 | 145739035 | c.2120G>T:p.C707F | NA | NA | NA | NA | NA |
| 16 | FANCA; ZNF276 | NM_000135 | exon 43; UTR3 | 89805058 | c.4319delA:p.Q1440fs | NA | NA | NA | NA | NA |
| 9 | FANCG | NM_004629 | exon 14 | 35074189 | c.1785C>A:p.S595R | NA | NA | NA | Polymorphism | Deleterious |
| 14 | SOS2 | NM_006939 | exon 18 | 50605450 | c.2838A>T:p.L946F | 0.000199681 (1000 g) | rs532833599 | NA | Pathogenic | Deleterious |
| 13 | BRCA2 | NM_000059 | exon 17 | 32936664 | c.7810C>A:p.L2604M | NA | NA | NA | Probably pathogenic | Deleterious |
| 19 | ERCC2 | NM_000400 | Splicing; intronic | 45871885 | exon5:c.360+3G>T | NA | NA | NA | NA | NA |
| 16 | ERCC4 | NM_005236 | exon 11 | 14041875 | c.2422G>T:p.A808S | NA | NA | NA | Pathogenic | Tolerated |
| 13 | ERCC5 | NM_000123 | exon 1 | 103498624 | c.8T>G:p.V3G | NA | NA | NA | Pathogenic | Deleterious |
| 16 | SLX4 | NM_032444 | exon 3 | 3656493 | c.742G>T:p.E248X | NA | NA | NA | Pathogenic | NA |
| 22 | NF2 | NM_000268 | exon 5; intronic | 30050687 | c.489G>T:p.L163F | NA | NA | NA | Pathogenic | Deleterious |
| 17 | NF1 | NM_001042492 | exon 25 | 29559152 | c.3259C>T:p.P1087S | NA | NA | NA | Pathogenic | Deleterious |
| exon 43 | 29664581 | c.6623C>A:p.A2208D | NA | NA | NA | Pathogenic | Deleterious |
ClinVar clinical significance of the variation, dbsnp Single Nucleotide Polymorphism Database, UMD predictor a high-throughput sequencing compliant system for pathogenicity prediction, SIFT sorting intolerant from tolerant; predicting amino acid changes that affect protein function
Fig. 3.
The protein–protein interaction network as analyzed by String software Version 11.0. The drawn edges represent the existence of different types of evidence used in predicting the associations
Sanger sequencing
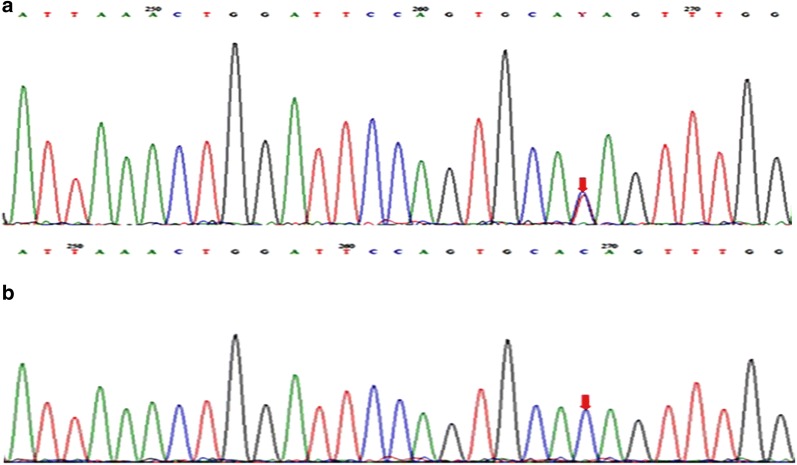
The mutation in the exon 10 of the MSH2 gene (exon 10; c.1552C>T; p.Q518X) was confirmed by Sanger sequencing (Fig. 4). We first confirmed it on the index case (CRCNab3) and then in all investigated first and second degree relatives (CRCNab2, CRCNab1, CRCNab4 and CRCNab5). The consequence of this mutation was a stop gain variant (p.Q518X). The global and the local Minor Allele frequencies (MAF) of this variant are illustrated in Table 3. In addition, neither p.L424V in POLE nor p.S478N in POLD1, were found by Sanger sequencing in investigated members.
Fig. 4.
Sanger sequencing chromatograms of MSH2 exon10 region: a control subject; b c.1552C>T in the index case (CRCNab3)
Table 3.
Lifestyle habits, phenotypes and Sanger sequencing results of screened family members
| Subject | Nab1 | Nab2 | Twins | Nab5 | Global MAF | Local MAF | Localization | ClinVar | eQTL p-value for colon tissu | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nab3 | Nab4 | |||||||||
| Age of onset | 47 | − | 33 | 35 | − | |||||
| Lifestyle habits (smoking and drinking) | Neither smoking nor alcohol intake | Neither smoking nor alcohol intake | +++ (consumption and exposure to smoking) +++ alcohol intake | ++ smoking | +++ smoking +++ alcohol intake | |||||
| Phenotype | CRC | Unaffected | CRC | Crohn | Unaffected | |||||
| Sanger sequencing: MSH2 (rs63750780) | M | M | M | M | M | NA | 0 | Exonic | Pathogenic | NA |
MAF minor allele frequency, Global MAF data from the 1000 genomes project, Local MAF from data available in our research laboratory of 59 whole exome sequencing of Tunisian subjects without CRC, eQTLs expression quantitative trait loci, M mutated (heterozygote), NA not available. Lifestyle habits of the investigated family members, including smoking and alcohol intake are summarized. ClinVar clinical significance of the variation
In silico prediction of the (p.Q518X) detected mutation on the MSH2/MSH6 dimerization
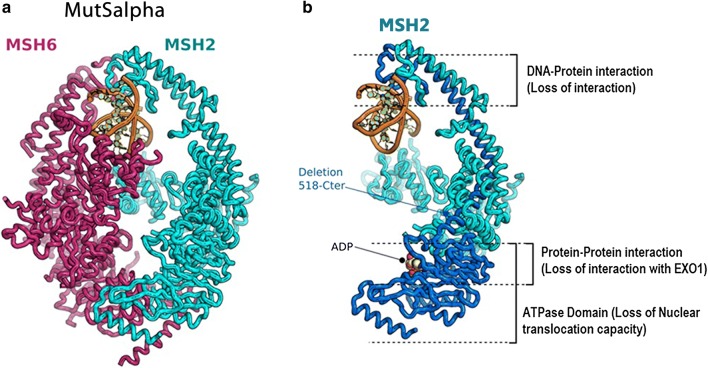
We have performed in silico prediction of the potential effect of this mutation on MSH2 protein structure and function. The Fig. 5 highlights the pathogenic effect of the identified MSH2 mutation on MSH2/MSH6 heterodimerization. MutSalpha consists of the association of the MSH6 and MSH2 which dimeric form is capable of recognizing the damaged DNA (Fig. 5a). We mapped the function segments downstream the stop codon insertion in dark blue (Fig. 5b). This results in loss of interaction between different regions within the heterodimer (protein–protein interaction loss, DNA–protein interaction loss and nuclear translocation activity loss). The structure result was performed in the bases of the MSH2/MSH6 complex structure of Warren et al. [21] (PDB code: 2O8B). This finding is in perfect concordance with the IHC pattern showing an MSH6 cytoplasmic accumulation and a loss of MSH2 expression.
Fig. 5.
Mapping the likely outcomes of the mutation (p.Q518X) on the structure of the MutSalpha DNA lesion recognition complex. a The association between MSH6 and MSH2 proteins which dimeric form is capable of recognizing the damaged DNA is called MutSalpha. b Corresponds to the MSH2 protein in which we mapped the function segments downstream the stop codon insertion in dark blue. This results in loss of interaction between the two proteins (MSH2/MSH6) and a default of heterodimer formation (protein-protein interaction loss, DNA-protein interaction loss and nuclear translocation activity loss)
Discussion
LS penetrance is highly variable and the reasons for this have not been fully elucidated. Peters et al. [22] affirmed that it remains critical that we stay on the path to uncover the complete genetic architecture of CRC to more fully understand the etiology of the disease. In Tunisian population, MMR germline mutations are responsible for at least 35.5% of CRC developed in patients with personal or familial history suggestive of Lynch syndrome [20]. In the only molecular Tunisian study by Moussa et al. [20], the entire coding regions, splice junctions and promoter regions of MLH1 and MSH2 were screened for the presence of point mutations. The following mutations in MSH2 and MLH1 were described in their investigated Tunisian LS families; MSH2 (p.Gln402X, p.Pro472ThrfsX4, p.Arg243Gln, p.Ser281X and p.Gly713ArgfsX4) and MLH1 (p.Ala111Asp, p.Gln197ArgfsX8, p.His718Tyr, p.Lys392SerfsX9, p.Arg226X and p.Glu153PhefsX8) [20]. MSH6 was analyzed but no mutation was founded. In this previous Tunisian study 64.5% (20/31) of families with suspicion of LS remain with undiscovered mutations in MMR genes. Thus, they suggest that other genes could predispose to non polyposis CRC. In this context, we described herein an interesting Tunisian family with strong history of colon cancer affecting three generations with a tumor spectrum specific to LS I form (Fig. 1). Genetic investigation using targeted sequencing DNA repair genes panel revealed, among detected variations, a single nucleotide substitution (c.1552C>T) in MSH2 in the proband (CRCNab3) and in his father (CRC Nab2). It was confirmed by Sanger sequencing (Fig. 2) and identified in the 47-years-old paternal uncle, diagnosed with a sigmoidien CRC and in three of the proband first degree relatives previously cited. This mutation is identified for the first time in a Tunisian LS family and was reported once by Fidalgo et al. [23] in an index case of LS Portuguese family. Fidalgo et al. [23] have confirmed the pathogenicity of this mutation by various approaches such as protein truncation test (PTT), single strand conformation polymorphism (SSCP), heteroduplex analysis (HA) and denaturing gradient gel electrophoresis (DGGE) as well [23]. The effect of this mutation is a premature stop codon (p.Q518X) which is already reported in InSiGHT variant databases (https://www.insight-group.org/variants/databases/) as a pathogenetic variant. The distribution of such rare mutation could be explained either by the only achieved molecular LS Tunisian study or by the scarcity of this mutation all over the world. The In silico prediction of the effect of this mutation on (MSH2·MSH6 heterodimer), crucial for MMR complex function [21, 24], revealed that its pathogenicity affects allosteric interactions between different regions within the heterodimer; loss of MSH2 ATPase Domain (loss of nuclear translocation capacity), loss of interaction with EXO1 and Loss of DNA–protein interaction. This will be translated in IHC expression profile by the loss of MSH2 nuclear expression and a cytoplasmic MSH6 accumulation. In almost all published articles using IHC analysis, the MSI phenotype is assigned following the loss of expression of MLH1 or MSH2 [20, 25, 26]. CRCNab3 phenotype was consistent with deficient MMR system (dMMR) linked to LS. The In situ functional effect of this mutation (c.1552C>T, p.Q518X) was confirmed by the obtained immunohistochemical pattern. Our IHC results are in concordance with molecular ones, supporting the evidence that MMR protein loss is explained notably by the pathogenic mutation in corresponding MMR gene. Moreover, IHC interpretation guidelines for cytoplasmic MMR staining bears no exact significance [27–29]. There are no data as yet to indicate that its presence is reflective of protein deficiency. Our results bring evidence that cytoplasmic staining could be taken into account to the evaluation of function loss within MSH2/MSH6 heterodimer. To the best of our knowledge, this is the first Tunisian study describing the effect of (c.1552C>T; p.Q518X) MSH2 mutation on MSH2/MSH6 complex heterodimerization, confirmed by IHC. Furthermore, it has been reported that in MSI cases, the presence of the BRAFV600E hotspot mutation excludes the diagnosis of LS, and the clinical utility of the combination of MMR and BRAF status is well established [30]. In this context, no somatic BRAFV600E mutation was detected in our investigated index case, confirming the LS.
In this work, the investigated family members share the same MSH2 pathogenic mutation (c.1552C>T, p.Q518X) with different phenotypes, suggesting hence, an important role of microenvironment and/or other DRGs mutations. The two CRC cases (CRCNab1 and CRCNab3) had both the MSH2 pathogenic mutation. CRCNab3 has alcohol and smoking habits that CRCNab1 has not. These two CRC patients showed some differences in colon tumor localization and age of disease onset. The 65 years-old unaffected mutation carrier father (CRC Nab2) had a healthy life style contrary to his 34 years-old unaffected mutation carrier son (CRC Nab5) who is a smoker and alcohol consumer.
Interestingly, MZ twins provide a model to investigate environmental effects on disease development and progression [31, 32]. The proband MZ twin (CRC Nab4) carried the MSH2 pathogenic mutation (c.1552C>T, p.Q518X) without alcohol habits. He has developed crohn disease (CD) at 35 years-old. It was already known that chronic inflammation creates a microenvironment suitable for the disease progression [33]. dosSantos [34], has identified that a pro-inflammatory state is the cornerstone in the association between CD and CRC, justifying the fact that CD might be a risk factor for CRC. She added that a family history of CRC is an important factor that doubles the risk of CRC in patients with CD. In our study, discordant twins’ habits allow us to suggest that they could directly or indirectly affect DNA changes independently of their mutational status. Alcohol consumption and cigarette smoking are considered as major risk factors for gastrointestinal cancer, including colorectal cancer [35]. The World Cancer Research Fund and the American Institute of Cancer Research suggests that excessive alcohol consumption enhance the risk of colorectal cancer. As a result of cumulative evidence from epidemiological studies, colorectal cancer has been listed as a pathology linked to alcohol intake and cigarette smoking [36]. This MZ twins discordance, pointed the important roles of environmental and modifiable factors in relation to gene–environment interactions in the prevention of CRC [37]. Studies of gene–environment interactions in families are crucial in providing potential insights for developing prevention strategies against CRC [38, 39]. Public health policies to prevent this cancer should include modification of alcohol intake habits, especially among individuals at increased risk [35, 40].
Carcinogenesis model in sporadic and hereditary CRC are based on the accumulation of mutations which is the critical determinant of tumorigenesis [41]. Currently, through genome-wide association studies, it has become possible to evaluate the role of common low-penetrance genetic modifiers and how they can affect disease expression that occurs both within families or individuals with similar MMR gene status [22]. Donald et al. [42], have conducted a meta-analysis to evaluate the role and effects of common low-penetrance genetic polymorphisms for a better understanding of their association with CRC risk in individuals belonging to LS families. They failed to uncover consistent evidence that LS phenotype is influenced by the effects of low penetrance modifiers. Weigl et al. [43] have identified that both family history and the identified genetic variants carry essential risk information and their combination provide great potential for CRC risk stratification. In this context, besides the pathogenic MSH2 mutation (c.1552C>T; p.Q518X) shared by the index case and his father, the Table 2 summarize other pinpointed pathogenic variants present on the index case not shared with his father.
It is widely recognized that environmental carcinogens induce DNA damage, which could in turn induce genomic instability [44]. The bulky DNA adducts generated by tobacco carcinogens are mainly repaired by nucleotide excision repair (NER). NER is the most common pathway for repairing bulky DNA lesions and maintaining genomic stability. Different key proteins are involved in this process including; ERCC2 (XPD) which accomplish 3′–5′ unwinding of the DNA strands of the damaged site, while the damaged DNA is excised at 5′ site by XPF (ERCC4)-ERCC1 heterodimer and at 3′ site by ERCC5 (XPG), which is an MSH2 and RECQL4 neighbor (Fig. 5). Aberrant expression of key NER factors alters NER capacity, thus threatening genomic stability and integrity [45]. In our study, we noted the following variants: ERCC2 (exon5:c.360+3G>T), ERCC4 (exon 11: c.2422G>T: p.A808S) and ERCC5 (exon 1: c.8T>G: p.V3G). Therefore, our identified alterations in the index case not shared with his father in NER pathway members could alter the efficacy of DNA repair and might enhance colorectal cancer risk. Only few studies have examined the contribution of SNPs in NER pathway genes to CRC risk [46, 47]. Our study is the first Tunisian one which highlights that variants in some members of the xeroderma pigmentosum (XP) genes family could play an important role in colorectal cancer increased risk.
Other cellular DNA repair pathways, such as base excision repair (BER), double-strand break repair (DSBR), and homologous recombination repair (HRR) also play important roles in the carcinogenesis process by repairing single strand and double strand DNA breaks induced by smoking, ionizing radiation, and other DNA damaging agents [47]. BRCA2 is a member of the HRR pathway, which restores the integrity of double-strand DNA breaks [48]. Inherited mutations in HRR genes have long been known to increase the risk of several cancers, including breast, ovarian, prostate and pancreatic cancers [49]. Risch et al. [50] reported that there is an increased risk for colon cancer in BRCA2 families. We identified a BRCA2 exon 17 variation (c.7810C>A: p.L2604M) in the index case.
BRCA2 is co-expressed with RecQ protein-like 4 (RECQL4) which is a key member of the RecQ family and plays an important role in the initiation of DNA replication, progression of stalled replication forks, and telomere maintenance, as well as in the repair of DNA DSB via the HRR pathway [51]. Mutations of the RECQL4 gene are associated with the rare type II Rothmund–Thomson syndrome, which has a propensity for osteosarcomas [52]. However, recent studies have shown that RECQL4 acts as a tumor-promotor in some cancers, such as prostate cancer, colorectal cancer, and breast cancer [52–55]. We detected (c.2120G>T: p.C707F) variation in the exon 13 of RECQL4. This is the first description of this variation in patients with CRC. Structure-specific endonuclease subunit (SLX4) encodes a Fanconi anemia-related protein that is required for repair of specific types of DNA lesions and critical for cellular responses to replication fork failure. Lee et al. [56] suggest that frameshift mutations of SLX4 may play a cancer-related role in limited cases of CRCs. We found an exon 3 SLX4 variation (c.742G>T: p.E248X).
NF1 which plays a role as a tumor suppressor gene [57] is co-expressed with RECQL4 and BRCA2. To date, the association between NF1 and adenocarcinoma of the gastrointestinal tract is thought to be casual. However, Li et al. [58] suggested that germline mutations in NF1 can occur in somatic cells and contribute to cancer development. Indeed, Seminog and Goldacre [59] observed that NF1 patients were at high risk of colon and recto-sigmoid junction cancer when compared with the general population. We detected NF1 variations in exon 25 (c.3259C>T: p.P1087S) and exon 43 (c.6623C>A:p. A2208D).
These actionable genes are not part of the recommended germline testing for individuals with familial CRC. The Fig. 3 showed their respectively protein–protein interactions, supporting the hypothesis that other variants unusually described in CRC might explain in part the phenotypic difference between the father and his son (CRCNab3). Thus, patients with multiple low penetrance SNPs could be experiencing an additive effect to increase CRC risk through gene–gene interactions. Confirmation of these identified variants by Sanger sequencing could be of important output regarding LS genetic profiling.
Conclusion
In overall, a better understanding of the genotype–phenotype correlation associated to LS may lead to implement a personalized oncogenetic counseling of individuals with particular mutational genetic profiles in terms of their risk management. Since, promoting a universal LS screening was the project aim of the International Mismatch Repair Consortium (IMRC), our study results are taking part from the conducted research projects in this field. Therefore, further studies are needed, with more particular attention to low penetrance modifier variants in order to better define the genotype–phenotype correlation and risk evaluation of colorectal carcinoma in LS context. Further conclusions regarding CRC-risk events should be based on a larger series of patients and families.
Additional file
Additional file 1. Targeted DNA repair genes panel list (87 genes).
Acknowledgements
The authors are extremely grateful to the patients whose participation made this work possible, to Dr. Houcemeddine Othman who participated to the in silico prediction and to Dr. Monia Ardhaoui who participated to the figure revision.
Abbreviations
- DNA
deoxyribonucleic acid
- gDNA
genomic DNA
- MAF
minor allele frequency
- NGS
next generation sequencing
- PCR
polymerase chain reaction
- SIFT
sorting intolerant from tolerant
- SNP
single nucleotide polymorphism
- VarAFT
variant annotation and filtering tool
- IGV
integrative genomic viewer
- MMR
mismatch repair
- BER
base excision repair
- DSBR
double-strand break repair
- HRR
homologous recombination repair
- NER
nucleotide excision repair
- XP
xeroderma pigmentosum
- IMRC
International Mismatch Repair Consortium
- CD
crohn disease
- MZ
monozygotic
- FFPE
formalin fixed paraffin embedded
- IHC
immunohistochemistry
- CRC
colorectal cancer
- LS
Lynch syndrome
- DRGs
DNA-repair genes
- MSI
microsatellite instability
Authors’ contributions
Study conception and design: AJG, HT, YH, LH, MA, SB and SA. Data acquisition: AJG, MK, MM, MM, LH, MA and AK. Analysis and interpretation of data: AJG, HT, MM and AK have analyzed and interpreted the patient clinic-pathological data. Bioinformatic analysis and networking: SE and HD and AJG. Contribution to the interpretation of the results YH, MK, IBA. Technical experiment: HY, NBJ and AM. Redaction of the full article: AJG. Involvement in the drafting of the manuscript: HT, MK, YH, SA and NM. Critical revision of the article: HT, SB, SA, MA and LH. Submission procedure: AJG. All authors read and approved the final manuscript.
Funding
This work was supported by the Tunisian Ministry of Public Health, the Tunisian Ministry of Higher Education and Scientific Research (LR16IPT05).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file.
Ethics approval and consent to participate
Written informed consents were obtained from all participants. Ethical approval according to the Declaration of Helsinki Principles was obtained from the biomedical ethics committee of Institut Pasteur de Tunis (2017/6A/I/Cancer colorectal/V1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amira Jaballah-Gabteni, Email: amirajaballah@gmail.com, Email: amira.jaballah@pasteur.utm.tn.
Haifa Tounsi, Email: haifa.tounsi@gmail.com, Email: Haifa.Tounsi@rns.tn.
Maria Kabbage, Email: maria.kabbage79@gmail.com.
Yosr Hamdi, Email: yosr.hamdi.82@gmail.com.
Sahar Elouej, Email: sahar.elouaj@univ-amu.fr.
Ines Ben Ayed, Email: inesbnayed@gmail.com.
Mouna Medhioub, Email: medhioub.mouna@yahoo.fr.
Moufida Mahmoudi, Email: mahmoudi.moufida@gmail.com.
Hamza Dallali, Email: hamza.dallali92@gmail.com.
Hamza Yaiche, Email: hamza.yaiche@pasteur.utm.tn.
Nadia Ben Jemii, Email: nadiabenjemii@gmail.com.
Afifa Maaloul, Email: jheyna@live.com.
Najla Mezghani, Email: najla.mezghani1980@gmail.com.
Sonia Abdelhak, Email: sonia.abdelhak@gmail.com, Email: sonia.abdelhak@pasteur.rns.tn.
Lamine Hamzaoui, Email: lamine015@yahoo.fr.
Mousaddak Azzouz, Email: mm.azzouz@rns.tn.
Samir Boubaker, Email: samir.boubaker@pasteur.rns.tn, Email: boubaker.samir@yahoo.fr.
References
- 1.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13(2):109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorans M, Dow E, Macrae FA, Winship IM, Buchanan DD. Update on hereditary colorectal cancer: improving the clinical utility of multigene panel testing. Clin Colorectal Cancer. 2018;17(2):e293–e305. doi: 10.1016/j.clcc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Liccardo R, De Rosa M, Duraturo F. Same MSH2 gene mutation but variable phenotypes in 2 families with lynch syndrome: two case reports and review of genotype-phenotype correlation. Clin Med Insights Case Rep. 2018;11:1179547617753943. doi: 10.1177/1179547617753943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv XP. Gastrointestinal tract cancers: genetics, heritability and germ line mutations. Oncol Lett. 2017;13(3):1499–1508. doi: 10.3892/ol.2017.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra N, Hall J. Identification of patients at risk for hereditary colorectal cancer. Clin Colon Rectal Surg. 2012;25(2):67–82. doi: 10.1055/s-0032-1313777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismael NE, El Sheikh SA, Talaat SM, Salem EM. Mismatch repair proteins and microsatellite instability in colorectal carcinoma (MLH1, MSH2, MSH6 and PMS2): histopathological and immunohistochemical study. Open Access Maced J Med Sci. 2017;5(1):9–13. doi: 10.3889/oamjms.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duraturo F, Cavallo A, Liccardo R, Cudia B, De Rosa M, Diana G, et al. Contribution of large genomic rearrangements in Italian Lynch syndrome patients: characterization of a novel alu-mediated deletion. Biomed Res Int. 2013;2013:219897. doi: 10.1155/2013/219897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero A, Garre P, Valentin O, Sanz J, Perez-Segura P, Llovet P, et al. Frequency and variability of genomic rearrangements on MSH2 in Spanish Lynch syndrome families. PLoS ONE. 2013;8(9):e72195. doi: 10.1371/journal.pone.0072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10(4):301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013;132(10):1077–1130. doi: 10.1007/s00439-013-1331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liccardo R, De Rosa M, Izzo P, Duraturo F. Novel implications in molecular diagnosis of Lynch syndrome. Gastroenterol Res Pract. 2017;2017:2595098. doi: 10.1155/2017/2595098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlDubayan SH, Giannakis M, Moore ND, Han GC, Reardon B, Hamada T, et al. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet. 2018;102(3):401–414. doi: 10.1016/j.ajhg.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellucci E, He T, Goldstein DY, Halmos B, Chuy J. DNA polymerase varepsilon deficiency leading to an ultramutator phenotype: a novel clinically relevant entity. Oncologist. 2017;22(5):497–502. doi: 10.1634/theoncologist.2017-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteban-Jurado C, Gimenez-Zaragoza D, Munoz J, Franch-Exposito S, Alvarez-Barona M, Ocana T, et al. POLE and POLD1 screening in 155 patients with multiple polyps and early-onset colorectal cancer. Oncotarget. 2017;8(16):26732–26743. doi: 10.18632/oncotarget.15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen AM, van Wezel T, van den Akker BE, Ventayol Garcia M, Ruano D, Tops CM, et al. Combined mismatch repair and POLE/POLD1 defects explain unresolved suspected Lynch syndrome cancers. Eur J Hum Genet. 2016;24(7):1089–1092. doi: 10.1038/ejhg.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsayed FA, Kets CM, Ruano D, van den Akker B, Mensenkamp AR, Schrumpf M, et al. Germline variants in POLE are associated with early onset mismatch repair deficient colorectal cancer. Eur J Hum Genet. 2015;23(8):1080–1084. doi: 10.1038/ejhg.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet. 2014;7:147–158. doi: 10.2147/TACG.S51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amira AT, Mouna T, Ahlem B, Raoudha A, Majid BH, Amel H, et al. Immunohistochemical expression pattern of MMR protein can specifically identify patients with colorectal cancer microsatellite instability. Tumour Biol. 2014;35(7):6283–6291. doi: 10.1007/s13277-014-1831-2. [DOI] [PubMed] [Google Scholar]
- 20.Moussa SA, Moussa A, Kourda N, Mezlini A, Abdelli N, Zerimech F, et al. Lynch syndrome in Tunisia: first description of clinical features and germline mutations. Int J Colorectal Dis. 2011;26(4):455–467. doi: 10.1007/s00384-010-1129-9. [DOI] [PubMed] [Google Scholar]
- 21.Warren JJ, Pohlhaus TJ, Changela A, Iyer RR, Modrich PL, Beese LS. Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell. 2007;26(4):579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Peters U, Bien S, Zubair N. Genetic architecture of colorectal cancer. Gut. 2015;64(10):1623–1636. doi: 10.1136/gutjnl-2013-306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fidalgo P, Almeida MR, West S, Gaspar C, Maia L, Wijnen J, et al. Detection of mutations in mismatch repair genes in Portuguese families with hereditary non-polyposis colorectal cancer (HNPCC) by a multi-method approach. Eur J Hum Genet. 2000;8(1):49–53. doi: 10.1038/sj.ejhg.5200393. [DOI] [PubMed] [Google Scholar]
- 24.Reyes GX, Schmidt TT, Kolodner RD, Hombauer H. New insights into the mechanism of DNA mismatch repair. Chromosoma. 2015;124(4):443–462. doi: 10.1007/s00412-015-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, Goossens M, Ouchene H, Hendriks-Cornelissen SJ, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–646. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Gray PN, Tsai P, Chen D, Wu S, Hoo J, Mu W, et al. TumorNext-Lynch-MMR: a comprehensive next generation sequencing assay for the detection of germline and somatic mutations in genes associated with mismatch repair deficiency and Lynch syndrome. Oncotarget. 2018;9(29):20304–20322. doi: 10.18632/oncotarget.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remo A, Fassan M, Lanza G, on behalf of AIFEG and GIPAD Immunohistochemical evaluation of mismatch repair proteins in colorectal carcinoma: the AIFEG/GIPAD proposal. Pathologica. 2015;107:104–109. [PubMed] [Google Scholar]
- 28.McCarthy AJ, Capo-Chichi JM, Spence T, Grenier S, Stockley T, Kamel-Reid S, et al. Heterogenous loss of mismatch repair (MMR) protein expression: a challenge for immunohistochemical interpretation and microsatellite instability (MSI) evaluation. J Pathol Clin Res. 2019;5(2):115–129. doi: 10.1002/cjp2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J, Kang Y, Lee YJ, Kim E, Ahn B, Lee E, et al. Comparison of the mismatch repair system between primary and metastatic colorectal cancers using immunohistochemistry. J Pathol Transl Med. 2017;51(2):129–136. doi: 10.4132/jptm.2016.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funkhouser WK, Jr, Lubin IM, Monzon FA, Zehnbauer BA, Evans JP, Ogino S, et al. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn. 2012;14(2):91–103. doi: 10.1016/j.jmoldx.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Allione A, Marcon F, Fiorito G, Guarrera S, Siniscalchi E, Zijno A, et al. Novel epigenetic changes unveiled by monozygotic twins discordant for smoking habits. PLoS ONE. 2015;10(6):e0128265. doi: 10.1371/journal.pone.0128265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcon F, Carotti D, Andreoli C, Siniscalchi E, Leopardi P, Caiola S, et al. DNA damage response in monozygotic twins discordant for smoking habits. Mutagenesis. 2013;28(2):135–144. doi: 10.1093/mutage/ges062. [DOI] [PubMed] [Google Scholar]
- 33.Freeman HJ. Colorectal cancer risk in Crohn’s disease. World J Gastroenterol. 2008;14(12):1810–1811. doi: 10.3748/wjg.14.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.dosSantos SCD, Barbosa LER. Crohn's disease: risk factor for colorectal cancer. J Coloproctol. 2017;37(1):55–62. doi: 10.1016/j.jcol.2016.06.005. [DOI] [Google Scholar]
- 35.Hughes LAE, Simons C, van den Brandt PA, van Engeland M, Weijenberg MP. Lifestyle, diet, and colorectal cancer risk according to (Epi)genetic instability: current evidence and future directions of molecular pathological epidemiology. Curr Colorectal Cancer Rep. 2017;13(6):455–469. doi: 10.1007/s11888-017-0395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho S, Shin A, Park SK, Shin HR, Chang SH, Yoo KY. Alcohol drinking, cigarette smoking and risk of colorectal cancer in the korean multi-center cancer cohort. J Cancer Prev. 2015;20(2):147–152. doi: 10.15430/JCP.2015.20.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiao SPK, Grayson J, Yu CH, Wasek B, Bottiglieri T. Gene environment interactions and predictors of colorectal cancer in family-based, multi-ethnic groups. J Personal Med. 2018 doi: 10.3390/jpm8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell PT, Curtin K, Ulrich CM, Samowitz WS, Bigler J, Velicer CM, et al. Mismatch repair polymorphisms and risk of colon cancer, tumour microsatellite instability and interactions with lifestyle factors. Gut. 2009;58(5):661–667. doi: 10.1136/gut.2007.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visser A, Vrieling A, Murugesu L, Hoogerbrugge N, Kampman E, Hoedjes M. Determinants of adherence to recommendations for cancer prevention among Lynch syndrome mutation carriers: a qualitative exploration. PLoS ONE. 2017;12(6):e0178205. doi: 10.1371/journal.pone.0178205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanella G, Archibugi L, Stigliano S, Capurso G. Alcohol and gastrointestinal cancers. Curr Opin Gastroenterol. 2018 doi: 10.1097/mog.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 41.Fearon ERVB. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 42.Donald N, Malik S, McGuire JL, Monahan KJ. The association of low penetrance genetic risk modifiers with colorectal cancer in lynch syndrome patients: a systematic review and meta-analysis. Fam Cancer. 2018;17(1):43–52. doi: 10.1007/s10689-017-9995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weigl K, Chang-Claude J, Knebel P, Hsu L, Hoffmeister M, Brenner H. Strongly enhanced colorectal cancer risk stratification by combining family history and genetic risk score. Clin Epidemiol. 2018;10:143–152. doi: 10.2147/CLEP.S145636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018 doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Li H, Sun L, Feng X, Wang Z, Yuan Y, et al. The differential expression of core genes in nucleotide excision repair pathway indicates colorectal carcinogenesis and prognosis. Biomed Res Int. 2018;2018:9651320. doi: 10.1155/2018/9651320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mucha B, Pytel D, Markiewicz L, Cuchra M, Szymczak I, Przybylowska-Sygut K, et al. Nucleotide excision repair capacity and XPC and XPD gene polymorphism modulate colorectal cancer risk. Clin Colorectal Cancer. 2018;17(2):e435–e441. doi: 10.1016/j.clcc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal N, Donald ND, Malik S, Selvendran SS, McPhail MJ, Monahan KJ. The association of low-penetrance variants in DNA repair genes with colorectal cancer: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2017;8(7):e109. doi: 10.1038/ctg.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mladenov E, Magin S, Soni A, Iliakis G. DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: cell cycle and proliferation-dependent regulation. Semin Cancer Biol. 2016;37–38:51–64. doi: 10.1016/j.semcancer.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107(12):2005–2009. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh DK, Popuri V, Kulikowicz T, Shevelev I, Ghosh AK, Ramamoorthy M, et al. The human RecQ helicases BLM and RECQL4 cooperate to preserve genome stability. Nucleic Acids Res. 2012;40(14):6632–6648. doi: 10.1093/nar/gks349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin J, Kwon YT, Varshavsky A, Wang W. RECQL4, mutated in the Rothmund–Thomson and RAPADILINO syndromes, interacts with ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Hum Mol Genet. 2004;13(20):2421–2430. doi: 10.1093/hmg/ddh269. [DOI] [PubMed] [Google Scholar]
- 53.Su Y, Meador JA, Calaf GM, De-Santis LP, Zhao Y, Bohr VA, et al. Human RecQL4 helicase plays critical roles in prostate carcinogenesis. Can Res. 2010;70(22):9207–9217. doi: 10.1158/0008-5472.CAN-10-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lao VV, Welcsh P, Luo Y, Carter KT, Dzieciatkowski S, Dintzis S, et al. Altered RECQ helicase expression in sporadic primary colorectal cancers. Transl Oncol. 2013;6(4):458–469. doi: 10.1593/tlo.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang H, Nie L, Chi Z, Liu J, Guo D, Lu X, et al. RecQL4 helicase amplification is involved in human breast tumorigenesis. PLoS ONE. 2013;8(7):e69600. doi: 10.1371/journal.pone.0069600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JH, An CH, Kim MS, Yoo NJ, Lee SH. Rare frameshift mutations of putative tumor suppressor genes CSMD1 and SLX4 in colorectal cancers. Pathol Res Pract. 2018;214(2):325–326. doi: 10.1016/j.prp.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Kim IY, Cho MY, Kim YW. Synchronous multiple colonic adenocarcinomas arising in patient with neurofibromatosis type 1. Ann Surg Treat Res. 2014;87(3):156–160. doi: 10.4174/astr.2014.87.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D, Ward K, Friedman E, Samowitz W, Robertson M, et al. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992;69(2):275–281. doi: 10.1016/0092-8674(92)90408-5. [DOI] [PubMed] [Google Scholar]
- 59.Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108(1):193–198. doi: 10.1038/bjc.2012.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Targeted DNA repair genes panel list (87 genes).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file.