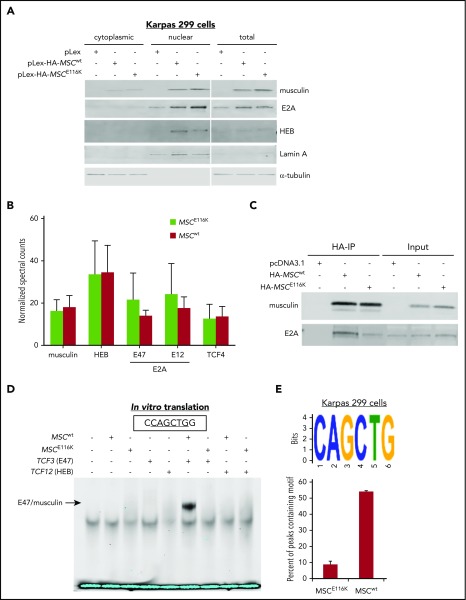
Figure 2.
MSCE116K disrupts musculin DNA binding but not protein interactions. (A) MSCwt and MSCE116K were expressed primarily in the nuclear fraction in Karpas 299 cells. MSCE116K is expressed more highly than MSCwt. A representative example from 3 independent experiments is shown. (B) Immunoprecipitation of HA-tagged MSCwt and MSCE116K in Karpas 299 cells, followed by mass spectrometry, revealed similar musculin:E-protein interactions. There was a slightly increased interaction between MSCE116K and E2A isoforms, although none of the differences was statistically significant. Data are shown as mean ± SD from 3 independent experiments. See also supplemental Table 5. (C) Western blot of immunoprecipitated musculin and E2A from experiment shown in (B). Input is also shown. (D) Electrophoretic mobility shift assay of proteins generated by in vitro translation of the indicated genes showed binding of E47/musculin heterodimers to the musculin-binding E-box motif CAGCTG in the presence of MSCwt but not MSCE116K. A representative example from 4 independent experiments is shown. (E) ChIP-seq for HA-tagged MSCwt and MSCE116K in Karpas 299 cells identified the CAGCTG E-box motif as the primary motif for MSCwt (E-value = 1.40E−176) but not for MSCE116K. See also supplemental Figure 8.