Figure 3.
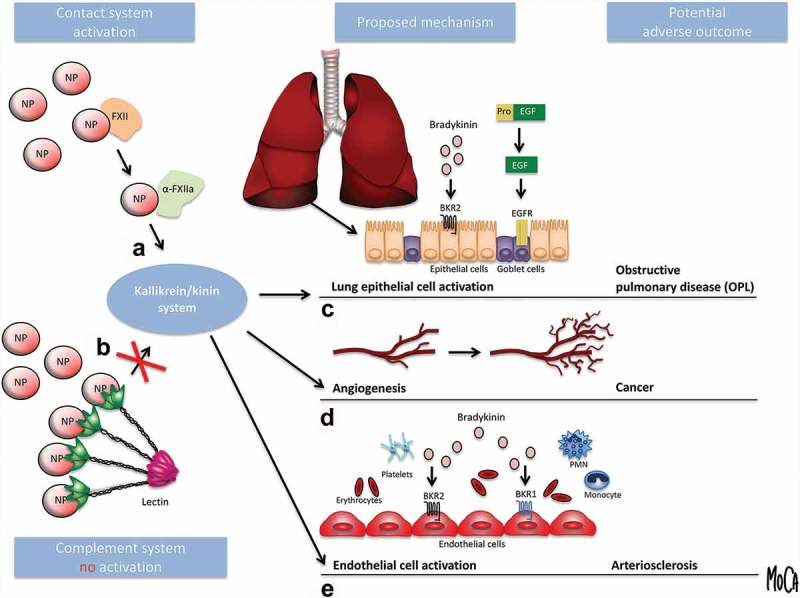
Model of nanoparticle (NP)-induced kallikrein/kinin system activation as a contributing factor in human disease. (A) FXII can get activated to α-FXIIa by direct interaction with NPs thereby initiating the kallikrein/kinin system. (B) The recognition molecules within the complement system (the lectins MBL, ficolins, and collectins within the lectin pathway and C1q within the classical pathway) are all multi-armed molecules which must encounter a conformational change by binding to closely located sites on a surface in order to induce activation of the complement cascade. NPs are too small to harbor more than on binding site and are therefore poor activators of complement. (C) Activated kallikrein can cause lung epithelial cell activation by kinin-dependent and kinin-independent mechanisms, e.g., by the generation of bradykinin and epidermal growth factor (EGF), which bind to their respective receptors (bradykinin receptors 1 and 2, BKR1 and BKR2; EGF receptor, EGFR). Ultimately, this activation may lead to obstructive pulmonary disease (OPD). (D) In addition, bradykinin, which is a potent inducer of angiogenesis, is implicated as a causative agent in cancer [58]. (E) Intravascular inflammation activates the patient’s endothelium, leading to loss of its anti-inflammatory and anti-thrombotic properties and the acquirement of a proinflammatory and prothrombotic phenotype, ultimately resulting in arteriosclerosis. The figure is from ref [57] and reproduced with permission from the publisher.
