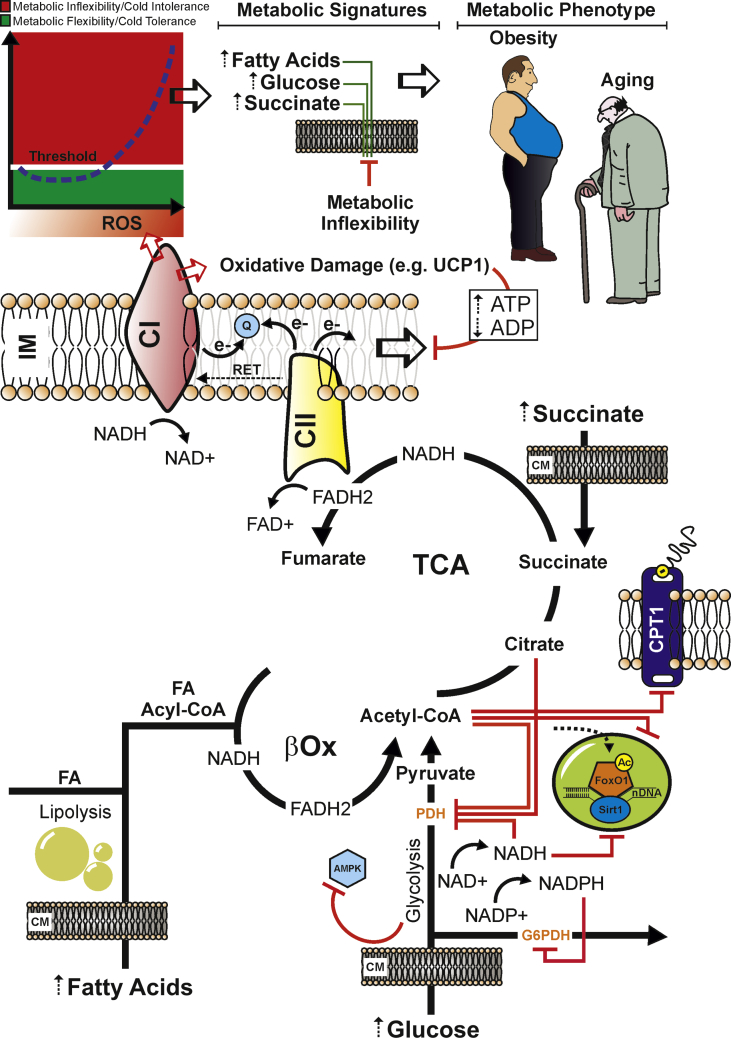
Figure 3.
Persistent nutrient overload promotes mitochondrial exhaustion and an uncontrolled ROS production that culminates in a systemic metabolic inflexibility. Dietary nutrient overload (e.g. fats and carbohydrates excess) enhances mitochondrial redox pressure (high NADH and FADH2) and electron flow. Under such stressful conditions, Q pool becomes over-reduced, and a high membrane potential drives the reverse transfer of electrons from Q to complex I (CI) in a process called reverse electron transport (RET). During RET electrons leak at CI generating a significant amount of superoxide. Overcoming the functional redox threshold induces mitochondrial oxidative damage in the redox sensitive proteins (e.g. UCP1), affecting the uncoupled respiration. This could increase the ATP levels, thus slowing-down (negative feed-back) the mitochondria electron transfer and proton pump. Nutrient overload also causes acetyl-CoA, citrate, NADH, FADH2, and NADPH accumulation, which allosterically inhibits the metabolic checkpoints, i.e. pyruvate dehydrogenase (PDH), glucose-6-posphate dehydrogenase (G6PDH), and carnitine palmitoyltransferase I (CPT1). Hence, AMPK could be phospho-inactivated and the nuclear FoxO1 could be hyperacetylated (as consequence of NAD+-dependent Sirt1 inhibition). This metabolic and molecular setting could promote the overwhelming of the redox threshold (red zone), which is causative of cold intolerance, metabolic inflexibility and mitochondrial substrate indecision. This condition leads to a systemic impairment of fuel utilization and elevation of circulating levels of glucose, fatty acids, and succinate, which represent typical metabolic hallmarks of obesity, type 2 diabetes and aging. IM: Inner Mitochondrial Membrane; CM: Cell Membrane.