Abstract
INTRODUCTION:
Cross-sectional data note lower levels of testosterone and sex hormone–binding globulin (SHBG) levels in men with nonalcoholic fatty liver disease (NAFLD). Whether sex hormone levels in young men are predictive of later risk of NAFLD is not known.
METHODS:
Among men in the prospective population-based multicenter Coronary Artery Risk Development in Young Adults study (mean age 50; n = 837), we assessed whether testosterone and SHBG levels measured at study year 10 (median age 35 years) were associated with prevalent NAFLD at study year 25. NAFLD was defined using noncontrast abdominal computed tomography (CT) scan after excluding other causes of hepatic steatosis. The association of testosterone and SHBG with prevalent NAFLD was assessed by logistic regression.
RESULTS:
Total testosterone levels in young men were inversely associated with subsequent prevalent NAFLD on unadjusted analysis (odds ratio [OR] 0.64,95% confidence interval 0.53–0.7, P< 0.001), although no longer significant after adjustment for year 10 metabolic covariates as well as change in metabolic covariates from years 10 to 25 (OR 0.99, 95% confidence interval 0.76–1.27). In contrast, there was a significant inverse association of SHBG with prevalent NAFLD, independent of testosterone and metabolic covariates (OR 0.68, OR 0.51–0.92, P = 0.013). On formal mediation testing, visceral adiposity was found to explain ~41.0% (95% confidence interval 27%–73%) of the association of lower SHBG with prevalent NAFLD.
CONCLUSIONS:
Lower levels of SHBG in young men are associated with increase in prevalent NAFLD in middle age, independent of comprehensive metabolic risk factors. SHBG may provide a novel marker of NAFLD risk in young men.
INTRODUCTION
Most circulating testosterone in men is bound to sex hormone–binding globulin (SHBG), a carrier protein produced by the liver. SHBG is a key protein involved in testosterone bioavailability, although growing literature also support potential non-androgen–related effects of SHBG on metabolic pathways (1). Cross-sectional studies demonstrate an inverse association of low testosterone and low SHBG with prevalent metabolic disease in men (2). Although longitudinal data have shown low SHBG to be associated with incident metabolic syndrome (3), these longitudinal data are inconsistent regarding the association of low testosterone with metabolic syndrome, particularly after adjustment for SHBG (4). Cross-sectional studies also demonstrate low SHBG and low testosterone levels in men with existing nonalcoholic fatty liver disease (NAFLD) (5), although the longitudinal association of either SHBG or testosterone with NAFLD has not been explored.
To address these knowledge gaps, we evaluated whether SHBG and testosterone obtained in healthy young men were associated with subsequent risk of prevalent NAFLD 15 years later. Importantly, these measurements were obtained among young men in their thirties, which reflects the time when testosterone and SHBG levels naturally begin to change in men. Whether sex hormone levels during this time period are associated with later risk of metabolic comorbidities, such as NAFLD, is not known. If identified, these data may support the role of testosterone and/or SHBG as early risk markers of NAFLD in men.
METHODS
Study population
We utilized data from the Coronary Artery Risk Development in Young Adults (CARDIA) study (6,7), and the CARDIA Male Hormone Study (8,9), an ancillary study to CARDIA. The CARDIA cohort is a multicenter observational cohort of cardiovascular disease in young black and white adults initially aged 18–30 years. Participants were recruited in 1985–1986 from 4 cities across the United States (US) (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) with the intent to enroll equal proportions of black and white participants. Subsequent follow-up occurred at years 2, 5, 7,10,15, 20, and 25, with a 72% retention rate of the surviving cohort by year 25. The initial cohort included n = 5,115 participants, of whom n = 2,327 (45%) were men. Participants were recruited by random digit dialing from total communities, census tract information, or healthcare plan as previously described (6). Institutional review board approval was obtained from all participating centers.
Cohort selection
From the initial cohort of CARDIA men (n = 2,327), we included those who underwent computed tomography (CT) quantification of hepatic steatosis at the year 25 follow-up examination (n = 1,379). Men without year 10 sex hormone measures (n = 365) and those with other potential causes of hepatic steatosis including alcohol use > 2 drinks/d (n = 146), self-report of hepatitis B or C (n = 19), those with HIV (n = 17), history of medication use associated with steatosis (amiodarone, methotrexate, valproic acid, or diltiazem) (n = 4), and/or those with exogenous testosterone use (n = 6) were excluded, for a final study sample of n = 837 men (Figure 1).
Figure 1.
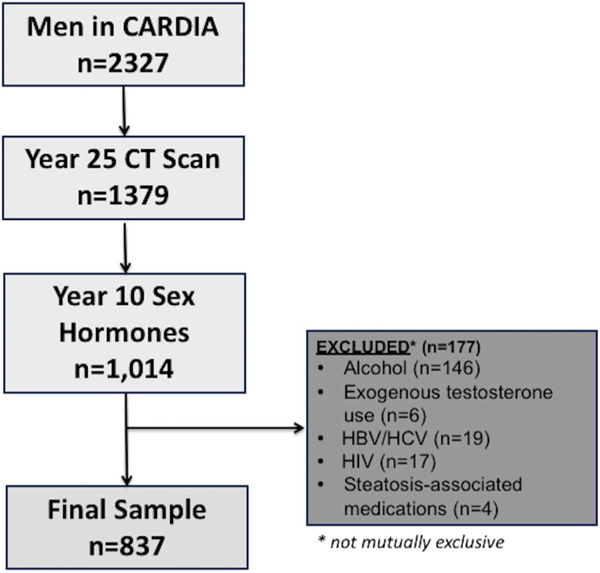
CARDIA participants meeting inclusion and exclusion criteria. Alcohol defined as >14 drinks per week. Steatosis-associated medications included amiodarone, methotrexate, diltiazem, and valproic acid. CARDIA, coronary artery risk development in young adults.
Hormone measurements
Our primary predictors were testosterone and SHBG levels performed at study year 10 (1995–1996) and secondarily from a smaller sample of men from study year 7 (1992–1993). These timepoints were selected as they capture men in the fourth decade of life when testosterone levels naturally begin to decline and conversely when SHBG levels begin to rise (10). Blood samples were collected by venipuncture and aliquots stored at –70 °C. Total testosterone was measured using radioimmunoassay; SHBG was measured by chemiluminescent enzyme immuno-metric assay (Immulite, Diagnostic Products Corporation, Los Angeles, CA) (9). Assay variability was monitored by including 10% blind quality control samples in each batch of samples analyzed. Intrabatch and interbatch variations were 12.3% and 11.2% for total testosterone, and 7.9% and 11.2% for SHBG. There was no association between time of blood draw and hormone levels (9). Bioavailable testosterone levels were calculated based on the Sodergard et al. method (11,12).
Hepatic steatosis
Year 25 noncontrast multidetector abdominal CT scan was performed using GE models 750HD (64) at the Birmingham site, GE LightSpeed VCT (64) in Oakland (GE Healthcare, Waukesha, Wisconsin), and Siemens Sensation 64 at the Chicago and Minneapolis sites (Siemens Medical Solutions, Erlangen, Germany). Quality control and image analysis were performed at a core reading center (Wake Forest University Health Sciences, Winston-Salem, North Carolina). CT diagnosis of hepatic steatosis was defined as liver attenuation (LA) of ≤40 Hounsfield Units (HUs). Mean LA values were determined from nine measurements on three CT slices of the right hepatic lobe. Previous studies using unenhanced CT scans have found LA values ≤40 HU to correlate with biopsy confirmed moderate-to-severe steatosis (>30%) (13–15). The characterization of LA in this cohort used a dedicated workflow within the previously described National Institute of Health’s Center for Information Technology Medical Image Processing, Analysis, and Visualization application (16). The interclass correlation coefficient between different readers on a random sample of 156 participants was 0.975 for LA, indicating high reproducibility of CT-measured LA in this cohort (16).
Covariates
Participant demographics, medical history, and alcohol use were obtained through standardized surveys as previously described in CARDIA (6). Race was defined as black or white by self-report. Medication use was obtained by self-report and subsequent verification of medications brought to study visits. Standard protocols were used for fasting serum collection and assays of plasma triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein (LDL) cholesterol, total cholesterol, serum glucose, and insulin levels (17,18). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the equation [fasting glucose (mmol/L) × fasting insulin (mU/L)]/22.5. These serum measures were analyzed from year 10 visit (referred to as baseline) and year 25 visit. Diabetes was defined as fasting glucose ≥126 mg/dL or 2-hour glucose tolerance test ≥200 mg/dL. Certified technicians performed anthropometric measurements including weight, height, and waist circumference using standardized protocols, and body mass index (BMI) calculated as weight (kg)/height (m)2. Waist circumference was measured midway between the iliac crest and bottom of the rib cage. Visceral adiposity tissue (VAT) volume was measured on the year 25 CT scan and reflects the sum of fat voxels within 10 mm set of slices centered at the L4–5 disk within the intraabdominal cavity. Interclass correlation coefficient for the inter-reader comparisons was 0.989 for VAT, and intra-reader and inter-reader errors were 2.4% and 6.7%, respectively, in 156 scans after blinded re-evaluation (19).
Statistical analyses
Year 10 and year 25 visit characteristics were compared using chi-square and Mann–Whitney U tests where appropriate. Logistic regression was used to evaluate the association of total testosterone and SHBG from year 10 with prevalent NAFLD at year 25. Estimated effects of total and bioavailable testosterone were reported per quartile to provide clinically relevant changes. SHBG was reported per s.d. (10 units). We also evaluated the association of sex hormones with prevalent NAFLD from a smaller sample of men with year 7 measurements, to assess for consistency of testosterone and SHBG estimates from a second timepoint during the fourth decade of life. Univariate and multivariate models assessed the association of covariates that were chosen a priori for clinical relevance and known associations with NAFLD. Covariates included age, race, and metabolic parameters including BMI, waist circumference, LDL, HDL, TG, and HOMA-IR. Given collinearity, waist circumference was selected over BMI in the multivariate model. To better capture the longitudinal effects of covariates on prevalent NAFLD, metabolic factors were analyzed at year 10 as well as change from years 10 to 25. The final multivariate model was developed using backward covariate selection, including those with 2-sided P values < 0.05 or strong biologic justification for inclusion. SHBG estimates were adjusted for testosterone and vice versa, given interdependent effects of these hormone levels on one another (20). Finally, to explore the potential mechanism by which SHBG or testosterone may contribute to NAFLD, we conducted formal testing for mediation of metabolic covariates using the STATA mediation package (21). This approach uses regression analyses to capture direct and indirect mediating effects of key variables and included visceral adiposity as measured on year 25 CT scan. Analyses were performed using STATA 15.1 (StataCorp, College Station, TX).
RESULTS
Study cohort characteristics
Of the 837 men meeting inclusion criteria, n = 115 (13.7%) had NAFLD by year 25, with median age at baseline of 35 years (inter-quartile range of 6). Compared to men without NAFLD, those with NAFLD were more likely to be White (69% vs 55%, P = 0.007) and to have worse baseline measures of most metabolic parameters, including higher TG (116 vs 83 mg/dL), higher HOMA-IR (2.5 vs 1.8), and greater waist circumference (98 vs 88 cm) (P values < 0.001) (Table 1). Bioavailable testosterone at year 10 was similar between those with and without NAFLD (P = 0.17) whereas total testosterone (4.7s vs 5.6 ng/mL) and SHBG (21.6 vs 27.4 ng/mL) were lower in men with NAFLD (P values < 0.001). Compared to men who were excluded from the study, those who met inclusion criteria had a lower prevalence of diabetes (1.0% vs 2.7%, P = 0.005) and were less likely to be Black (43% vs 54%, P < 0.001). Statistically significant but clinically similar differences in the remaining metabolic parameters are shown in Supplemental Table 1 (see Supplemental Digital Content, http://links.lww.com/AJG/A56).
Table 1.
Cohort characteristics (year 10) by NAFLD status at year 25 (n = 837)
| Non-NAFLD (n = 722) |
NAFLD (n = 115) |
P value | |
|---|---|---|---|
| Age, median y (IQR) | 35.0 (6.0) | 36.0 (7.0) | 0.038 |
| Race, n (%) | |||
| Black | 322 (44.6) | 36(31.3) | 0.007 |
| White | 400 (55.4) | 79 (68.7) | |
| BMI, median kg/cm2 (IQR) | 26.3 (5.5) | 29.0 (7.0) | <0.001 |
| Waist circumference, | 87.8(13.8) | 97.5(16.8) | <0.001 |
| median cm (IQR) | |||
| Totalcholesterol, | 180 (47) | 185 (45) | 0.009 |
| median mg/dL (IQR) | |||
| HDL, median mg/dL (IQR) | 44 (15) | 39(11) | <0.001 |
| LDL, median mg/dL (IQR) | 113(43) | 119(39) | 0.032 |
| Triglycerides, median mg/dL (IQR) | 83(69) | 116(95) | <0.001 |
| Diabetes history, n (%) | 12(1.7) | 1 (0.9) | 0.523 |
| HOMA-IR | 1.8 (1.1) | 2.5 (1.7) | <0.001 |
| Sex hormone-binding globulin, | 27.4 (14.9) | 21.6(11.0) | <0.001 |
| median nmol/L (IQR) | |||
| Totaltestosterone, | 5.6 (2.3) | 4.7 (1.9) | <0.001 |
| median ng/mL (IQR) | |||
| Bioavailable testosterone, | 2.8 (1.2) | 2.6 (1.1) | 0.168 |
| median ng/mL (IQR) | |||
IQR, interquartile range.
On unadjusted analysis, total testosterone was inversely associated with prevalent NAFLD at year 25 (odds ratio [OR] 0.64,95% CI [confidence interval] 0.53–0.77, P < 0.001) although no longer significant on the fully adjusted model including SHBG, year 10 metabolic covariates, and change in metabolic covariates from years 10 to 25 (OR 0.99, 95% CI 0.76–1.27, P = 0.909) (Table 2). Supplemental Table 2 (see Supplemental Digital Content, http://links.lww.com/AJG/A56) demonstrates the relative change in these estimates on progressive covariate adjustment. Similar findings were observed when analyzing bioavailable testosterone levels, with an inverse association with prevalent NAFLD noted on unadjusted analysis (OR 0.84,95% CI 0.70–1.0, P = 0.055), although attenuated in the fully adjusted model (OR 0.99,95% CI 0.80–1.24, P = 0.963).
Table 2.
Association of year 10 SHBG and testosterone levels with prevalent NAFLD at year 25 (n = 837)
| Univariate analysis |
Multivariate analysisb |
|||
|---|---|---|---|---|
| Characteristica | OR (95% CI) | P value | AOR (95% CI) | P value |
| SHBG (per 10 units) | 0.55 (0.44–0.69) | <0.001 | 0.68 (0.51–0.92) | 0.013 |
| Testosterone (per quartile) | 0.64 (0.53–0.77) | <0.001 | 0.99 (0.76–1.27) | 0.909 |
| Age (per y) | 1.06(1.00–1.12) | 0.059 | 1.05 (0.98–1.123) | 0.138 |
| White versus black race | 1.77(1.16–2.7) | 0.008 | 2.27(1.34–3.85) | 0.002 |
| CARDIA center | 1.35(1.12–1.63) | 0.002 | 1.53(1.21–1.94) | <0.001 |
| BMI (per 5 kg/m2) | 1.77(1.47–2.11) | <0.001 | — | — |
| Δ BMI (per 5 kg/m2) | 2.76 (2.02–3.76) | <0.001 | — | — |
| Waist circumference (per 1 cm) | 1.06(1.05–1.08) | <0.001 | 1.04(1.02–1.07) | 0.002 |
| Δ Waist circumference (per 1 cm) | 1.10(1.08–1.13) | <0.001 | 1.11 (1.07–1.15) | <0.001 |
| Triglycerides (per 20 mg/dL) | 1.10(1.06–1.15) | <0.001 | — | — |
| Δ Triglycerides (per 20 mg/dL) | 1.04(1.00–1.08) | 0.059 | — | — |
| LDL (per 10 mg/dL) | 1.05(1.00–1.11) | 0.068 | — | — |
| Δ LDL (per 10 mg/dL) | 0.91 (0.86–0.96) | 0.001 | — | — |
| HDL (per 5 mg/dL) | 0.75 (0.67–0.83) | <0.001 | 0.87 (0.77–1.00) | 0.061 |
| Δ HDL (per 5 mg/dL) | 0.98 (0.96–1.00) | 0.026 | 0.91 (0.78–1.07) | 0.268 |
| HOMA-IR (per unit) | 1.26(1.15–1.37) | <0.001 | 1.21 (1.05–1.38) | 0.006 |
| Δ HOMA-IR (per unit) | 1.22(1.15–1.31) | <0.001 | 1.09(1.02–1.16) | 0.013 |
95% CI, confidence interval; BMI, body mass index; CARDIA, coronary artery risk development in young adults; HDL, high density lipoprotein; HOMA-IR, Homeostatic Model of Assessment of Insulin Resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; SHBG, sex hormone–binding globulin.
Δ values reflect unit change from years 10 to 25 to capture potential confounding effects of metabolic parameters over time.
Waist circumference selected over BMI to capture visceral adiposity.
AOR, adjusted odds ratio; LDL and triglycerides not significant on adjusted analysis.
In contrast to testosterone, SHBG remained inversely associated with prevalent NAFLD on unadjusted (OR 0.55, 95% CI 0.44–0.69, P < 0.001) and fully adjusted models (OR 0.68, 95% CI 0.51–0.0.92, P = 0.013) (Table 2). Changes in SHBG estimates with progressive covariate adjustment are also shown in Supplemental Table 2 (see Supplemental Digital Content, http://links.lww.com/AJG/A56). Figure 2 demonstrates NAFLD prevalence across quartiles of year 10 SHBG, with approximately 24% of men with the lowest quartile of SHBG having NAFLD, as compared to < 6% of men with the highest SHBG levels, P < 0.001 (Figure 2). SHBG estimates also remained consistent on adjusted analysis in a smaller subset of men (n = 730) with available sex hormone measurements from year 7 (OR 0.70,95% CI 0.52–0.94, P = 0.019), at which time men were on average 32 years of age.
Figure 2.
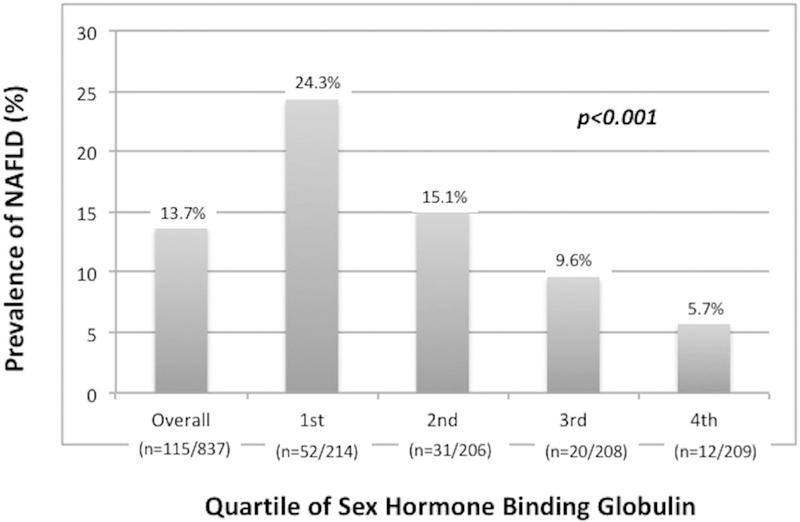
Prevalence of NAFLD by sex hormone-binding globulin levels. NAFLD, nonalcoholic fatty liver disease.
To further explore the mechanism by which SHBG may contribute to NAFLD, we conducted formal testing for mediation using year 25 covariates in the fully adjusted models including baseline and change in metabolic variables (21). Visceral adipose tissue volume was found to be a significant mediator, with 41.0% (95% CI 27%–73%) of the observed association of SHBG with prevalent NAFLD attributed to this factor. Year 25 HDL (25% mediation effect, 95% CI 14%–46%) and HOMA-IR (19% effect, 95% CI 7%–36%) were also statistically significant mediators, although with smaller estimated mediating effects.
DISCUSSION
Using data from the large prospective CARDIA cohort study, we identified an association of SHBG measured in healthy young men with risk of prevalent NAFLD in midlife. This association was independent of a comprehensive panel of metabolic parameters including anthropomorphic measures, lipids, and insulin resistance. In contrast to our findings for SHBG, the association of low testosterone with NAFLD in men did not persist after adjusting for metabolic covariates.
A growing body of literature supports sexually dimorphic associations of testosterone with NAFLD in men compared to women (2), although longitudinal data in men are limited, including lack of adjustment for SHBG. Cross-sectional data from the Multi-Ethnic Study of Atherosclerosis cohort found higher testosterone to be associated with NAFLD in women (22), consistent with our previous work among women in CARDIA (23), whereas similar to the current study, they found only SHBG, but not lower testosterone, to be associated with NAFLD in men. Whether low SHBG is a better marker of NAFLD in men is possible, although randomized controlled trials of testosterone replacement in men do note improvement in metabolic parameters, including insulin resistance, LDL levels, and VAT (24–26). Such data support a more direct effect of testosterone on metabolic disease in men and the need for ongoing investigation of testosterone’s role in NAFLD. The use of an immunoassay for testosterone measurements, rather than the more sensitive liquid chromatography–mass spectroscopy method, may have contributed to our inability to detect a longitudinal association of total testosterone and NAFLD in men. However, data from the longitudinal Framingham Heart Study, which did use liquid chromatography-mass spectroscopy, found that low testosterone was only associated with concurrent metabolic syndrome, whereas the longitudinal association of testosterone with incident metabolic syndrome was no longer apparent after adjustment for SHBG, insulin resistance, and BMI. In contrast, there was a strong and persistent association of low SHBG with incident metabolic syndrome more than 6 years later (3).
Most studies linking SHBG to NAFLD have been cross-sectional in design, analyzing SHBG levels obtained at the time of NAFLD assessment (2). The association of low SHBG with NAFLD is thought to relate to the inhibitory effects of circulating insulin (27), inflammatory cytokines, and hepatic lipogenesis on SHBG production by the liver (28,29). The current study expands upon existing literature by demonstrating the association of lower SHBG in otherwise healthy young men, on their subsequent risk of prevalent NAFLD. Although the time of NAFLD onset cannot be confirmed in our cohort, it is unlikely that men in their 30s without existing metabolic comorbidities had NAFLD at baseline, particularly during a time period before the obesity and diabetes epidemics (30). The consistency of SHBG estimates in men from 2 separate timepoints during their 30s lends confidence to these findings and suggests that SHBG may have a role as a novel NAFLD risk marker in young men.
The mechanism by which SHBG could promote NAFLD in humans is not fully understood, and whether the association of SHBG reflects an epiphenomenon or more direct role of SHBG on NAFLD pathogenesis is not known. Recent data in mouse models have shown that overexpression of SHBG in mice reduces hepatic triglyceride content by way of reducing key lipogenic enzyme activity (1). These authors also found that treatment of mice using exogenous SHBG reduced peroxisome proliferator-activated receptor gamma activity, thereby directly regulating hepatic lipogenesis. In the current study, we used formal mediation testing (21) to begin exploring factors that may play a causal role along the pathway from SHBG to NAFLD in men. In these analyses, we identified a significant mediating effect of visceral adiposity, and to a lesser degree, that of low HDL and insulin resistance. Although mechanistic studies are needed in humans, our data support the growing literature on a potential broader role of SHBG in the development of metabolic disease.
The current study has important strengths and some notable limitations. The CARDIA cohort includes a large, well-characterized population of white and black men with detailed metabolic parameters followed for more than 2 decades, although lack of reported Hispanic ethnicity limits generalizability to these populations. Given the lack of liver pathology, the association of hormones with nonalcoholic steatohepatitis (e.g., NASH) in men could not be addressed. CT imaging was also unavailable at baseline; therefore, the timing of NAFLD development could not be ascertained. However, as noted above, it is not likely that young men without metabolic disease had NAFLD at baseline. We also note the lower prevalence of NAFLD in our cohort than what may be expected in the general population. We defined NAFLD by LA cutoffs that were based on previous data showing excellent specificity, though lower sensitivity, for histologically confirmed NAFLD (13). Higher specificity minimizes the effect of measurement bias (31), although this comes at a cost of decreased NAFLD detection for those with mild degrees of steatosis. Finally, we used a dedicated statistical program to evaluate potential mediators of the association between SHBG and NAFLD in men, which helps to support the important role of visceral adiposity along the causal pathway from testosterone to NAFLD in men.
In conclusion, low SHBG levels measured in healthy young black and white men were associated with higher risk of prevalent NAFLD 15 years later, independent of comprehensive measures of metabolic cofactors. In contrast, the longitudinal association of testosterone with NAFLD risk was not apparent in the current study. Our data support the need for additional mechanistic studies evaluating the role of SHBG on NAFLD development and support the potential role of SHBG as a risk marker for NAFLD in young men.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
✔ Testosterone and SHBG levels are decreased in men with diabetes, visceral adiposity, and NAFLD.
✔ It is not known whether low hormone levels in healthy young men are associated with later risk of NAFLD.
WHAT IS NEW HERE
✔ SHBG levels in healthy young men were inversely associated with prevalent NAFLD in midlife.
✔ In contrast, testosterone was no longer associated with NAFLD, after adjusting for metabolic confounders.
Acknowledgments
Financial support: The CARDIA Study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), Johns Hopkins University School of Medicine (HHSN268200900041C), and Vanderbilt University Medical Center (R01HL098445). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging. This article has been reviewed by CARDIA for scientific content. The following authors are supported by the National Institutes of Health (NIH): Dr. Sarkar (K23-DK111944), Dr. Van-Wagner (K23-HL136891), and Dr. Carr (R01-HL-098445). The CWS is supported by the National Heart, Lung, and Blood Institute grant R01-HL065611 and contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-4805. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICTS OF INTEREST
Guarantor of the article: Monika Sarkar, MD, MAS.
Potential competing interests: None.
REFERENCES
- 1.Saez-Lopez C, Barbosa-Desongles A, Hernandez C, et al. Sex hormone-binding globulin reduction in metabolic disorders may play a role in NAFLD development. Endocrinology 2017;158:545–59. [DOI] [PubMed] [Google Scholar]
- 2.Jaruvongvanich V, Sanguankeo A, Riangwiwat T, et al. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann Hepatol 2017;16:382–94. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Jasjua GK, Pencina M, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: The framingham heart study. Diabetes Care 2011;34:2464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh JY, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of type 2 diabetes in older men and women: The Rancho Bernardo study. Diabetes Care 2002;25:55–60. [DOI] [PubMed] [Google Scholar]
- 5.Seo NK, Koo HS, Haam JH, et al. Prediction of prevalent but not incident non-alcoholic fatty liver disease by levels of serum testosterone. J Gastroenterol Hepatol 2015;30:1211–6. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 7.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials 1991;12: 1S–77S. [DOI] [PubMed] [Google Scholar]
- 8.Gapstur SM, Kopp P, Gann PH, et al. Changes in BMI modulate age-associated changes in sex hormone binding globulin and total testosterone, but not bioavailable testosterone in young adult men: The CARDIA male hormone study. Int J Obes (Lond) 2007;31:685–91. [DOI] [PubMed] [Google Scholar]
- 9.Gapstur SM, Gann PH, Kopp P, et al. Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 2002;11:1041–7. [PubMed] [Google Scholar]
- 10.Allan CA, McLachlan RI. Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol (Oxf) 2004;60:653–70. [DOI] [PubMed] [Google Scholar]
- 11.Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–10. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: Use of CT for quantitative and qualitative assessment. Radiology 2006;239:105–12. [DOI] [PubMed] [Google Scholar]
- 14.Zeb I, Li D, Nasir K, et al. Computed tomography scans in the evaluation of fattyliver disease in a population based study: The multi-ethnic study of atherosclerosis. Acad Radiol 2012;19:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol 2007;188:1307–12. [DOI] [PubMed] [Google Scholar]
- 16.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The coronary artery risk development in young adults study. Atherosclerosis 2014;235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CE, Funkhouser E, Raczynski JM, et al. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary artery risk development in young adults. Am J Epidemiol 1996;144:247–54. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: The CARDIA study. Ann Epidemiol 1996;6:235–45. [DOI] [PubMed] [Google Scholar]
- 19.VanWagner LB, Wilcox JE, Colangelo LA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology 2015;62:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyawali P, Martin SA, Heilbronn LK, et al. Cross-sectional and longitudinal determinants of serum sex hormone binding globulin (SHBG) in a cohort of community-dwelling men. PLoS One 2018;13:e0200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks R, Tingly D. Causal mediation analysis. Stata J 2011;11:1–15. [Google Scholar]
- 22.Lazo M, Zeb I, Nasir K, et al. Association between endogenous sex hormones and liver fat in a multiethnic study of atherosclerosis. Clin Gastroenterol Hepatol 2015;13:1686–93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar M, Wellons M, Cedars MI, et al. Testosterone levels in premenopausal women are associated with nonalcoholic fatty liver disease in midlife. Am J Gastroenterol 2017;112:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 2016; 39:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906. [DOI] [PubMed] [Google Scholar]
- 27.Plymate SR, Matej LA, Jones RE, et al. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 1988;67:460–4. [DOI] [PubMed] [Google Scholar]
- 28.Simo R, Barbosa-Desongles A, Lecube A, et al. Potential role of tumor necrosis factor-alpha in downregulating sex hormone-binding globulin. Diabetes 2012;61:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simo R, Barbosa-Desongles A, Saez-Lopez C, et al. Molecular mechanism of TNFalpha-induced down-regulation of SHBG expression. Mol Endocrinol 2012;26:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: The third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol 1977; 105:488–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


