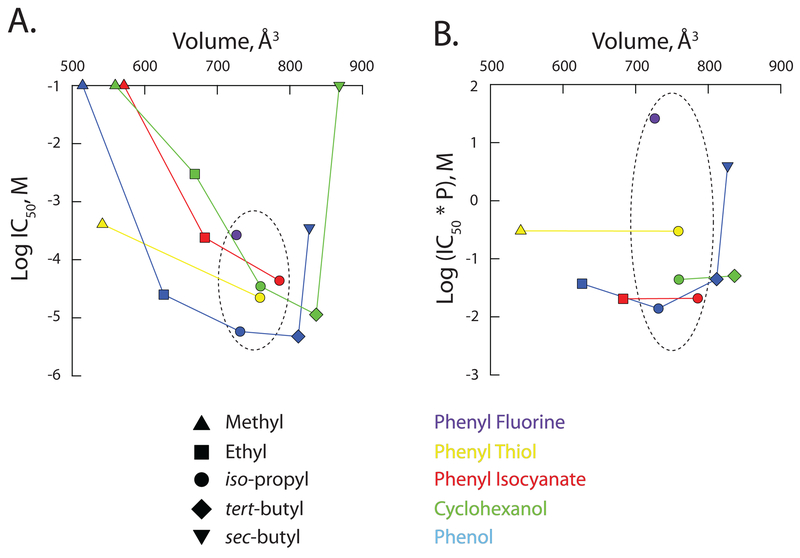
Figure 4: Inverse agonist potency of 2,6-di-alkyl phenyl analogues as a function of molecular volume.
The observed aqueous IC50 (A) and the
partition-corrected IC50 (B) plotted as a function of
the calculated molecular volume. Except where no inflection was observable in
the concentration response curve, IC50’s were determined from
the fits shown in Figure 3 (see also Table 3). For ineffective molecules, the
IC50 was set equal to 100 mM. The partition-corrected
IC50 is the aqueous IC50 multiplied by the calculated
partition coefficient, P (as per
Tables 1 and 2). In both A and B, the
dashed ellipse encircles the data for the iso-propyl family of
reagents. As their IC50s are at best ill-defined, values for DM-P
( ), DM-PIC
(
), DM-PIC
( ), DM-CH
(
), DM-CH
( ), DE-CH
(
), DE-CH
( ) and DSBCH
(
) and DSBCH
( ) are omitted from
B.
) are omitted from
B.