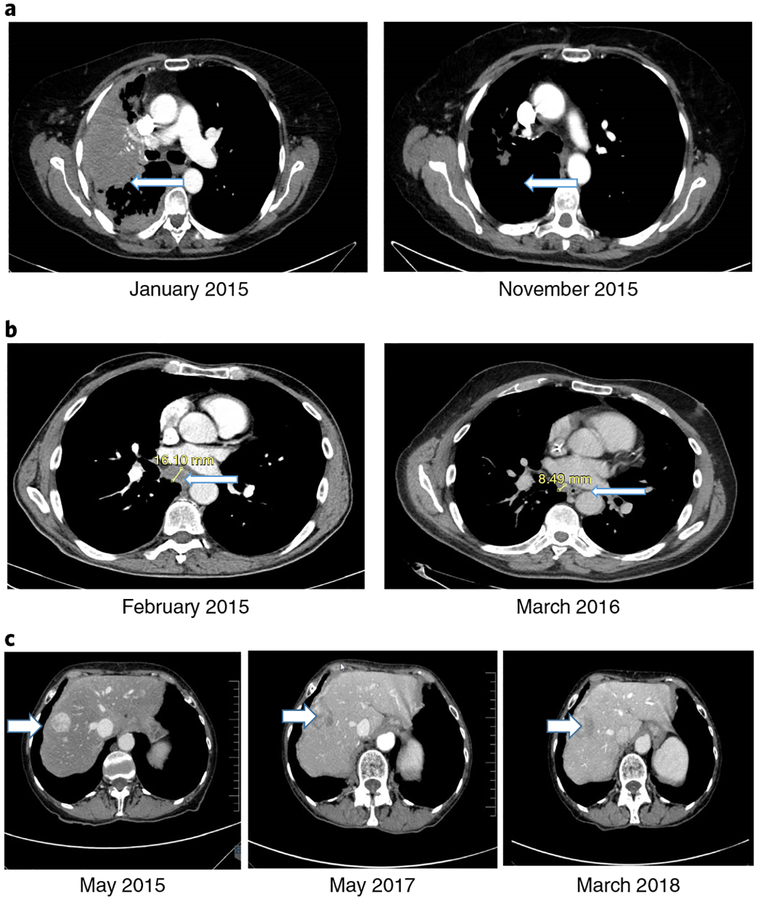
Fig. 2 |. Examples of exceptional responses with CT scans.
a, A 72-year-old woman with non-small-cell lung adenocarcinoma progressing after erlotinib and pemetrexed enrolled on the WINTHER trial (November 2014). NGS matches found a EGFR T790M mutation (which leads to a resistance to the drugs available at the time). The WINTHER CMC recommended the EGFR small-molecule inhibitor afatinib with the antibody cetuximab, according to data showing responses with this combination16. (Osimertinib (an EGFR inhibitor targeting EGFR T790M) was not yet approved.) Outcome refers to a CR (PFS of 13 months). The left panel is pre-treatment and shows a large lung mass (arrow). The right panel shows tumor resolution (arrow). b, A 68-year-old man with progressive metastatic colorectal cancer after Xelox (capecitabine and oxaliplatin) and FOLFIRI (folinic acid, 5-fluoruracil, irinotecan)-cetuximab. NGS matches found a MSH6 mutation (a mismatch repair gene alteration causing microsatellite instability). The WINTHER CMC recommended pembrolizumab, according to data newly emerging at the time (and later validated17) regarding checkpoint inhibitor efficacy in a mismatch repair gene defect setting. Pembrolizumab was initiated in March 2015. Outcome refers to a PR (PFS of >36 months). The left panel shows baseline mediastinal adenopathy (arrow). The right panel shows mediastinal adenopathy regression (arrow). c, A 69-year-old woman with a well-differentiated, neuroendocrine, small gut tumor and peritoneal metastasis underwent debulking surgery and received lanreotide (a long-acting somatostatin analog) for residual disease (April 2011 to June 2012). She then developed bowel obstruction (due to peritoneal progression), which required surgery. The somatostatin analog continued until April 2014, when progression to the liver occurred. Axitinib (on a clinical trial) was given for 10 months before progression. In April 2015, she enrolled on the WINTHER trial. NGS found no DNA alterations; RNA matches revealed AKT2 and AKT3 overexpression. The WINTHER CMC recommended an mTOR inhibitor. Everolimus was started (May 2015). Outcome refers to prolonged disease stabilization (PFS of >34 months). (Everolimus was later approved for this indication in 2016.) The left panel shows baseline liver metastases (arrow). The middle panel shows stable liver metastases (arrow) at 1 yr. The right panel shows ongoing stable liver metastases (arrow) at >34 months. The examples of the exceptional responses shown in a-c can be found in Supplementary Table 4 (ID156, ID183 and ID203, respectively).