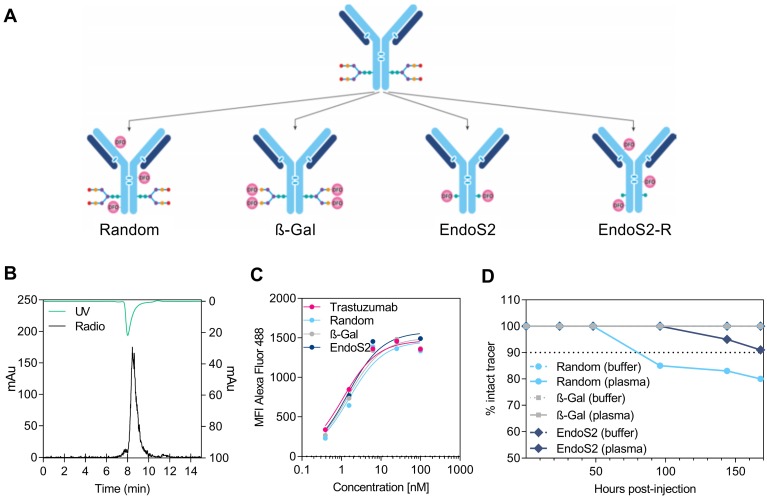
Figure 1.
Radiochemistry and in vitro evaluation of 89Zr-DFO-trastuzumab. (A) Schematic illustration of the 4 alternate ways of DFO conjugation to trastuzumab: (1) randomly on lysine residues (random), site-specifically on enzymatically treated glycans using either (2) β-galactosidase (β-Gal) or (3) endoglycosidase S2 (endoS2), or randomly on lysine residues after endoS2 modification (endoS2-R). (B) Representative HPLC chromatogram of endoS2 modified 89Zr-DFO-trastuzumab after PD10 purification. (C) Median fluorescent intensity (MFI) of SK-OV-3 cells incubated with increasing concentrations of unlabeled, random, β-Gal and endoS2 modified trastuzumab. (D) Radio-TLC analysis of tracer stability in plasma and buffer up to 168 hours post-labeling.