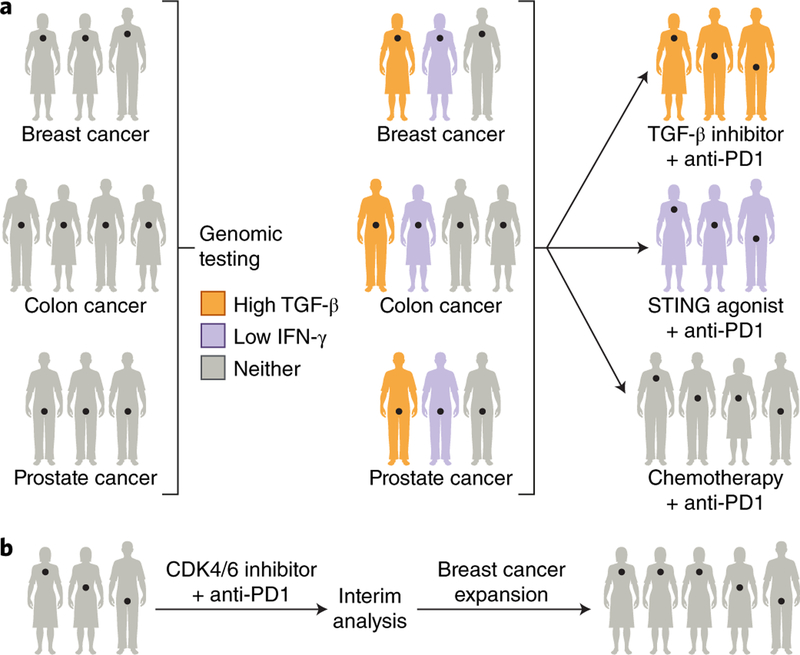
Fig. 3 |. Trial designs to overcome checkpoint blockade resistance.
a, A basket trial matches therapeutic agents based on genomic alterations irrespective of tumor site of origin. In the illustrated example, breast, colorectal, and prostate tumors are tested for TGF-β and IFN-γ transcriptomic signatures. Those patients whose tumors possess high TGF-β or low IFN-γ signatures are treated with TGF-β inhibitors or STING agonists, respectively, in addition to anti-PD-1 therapy, while tumors without genomic targets are treated with chemotherapy and anti-PD-1 therapy. b, An adaptive trial design employs interim analyses to make modifications, such as expansion of patient cohorts with more promising responses, illustrated in the figure as an expansion of the breast cancer cohort. Credit: Debbie Maizels/Springer Nature.