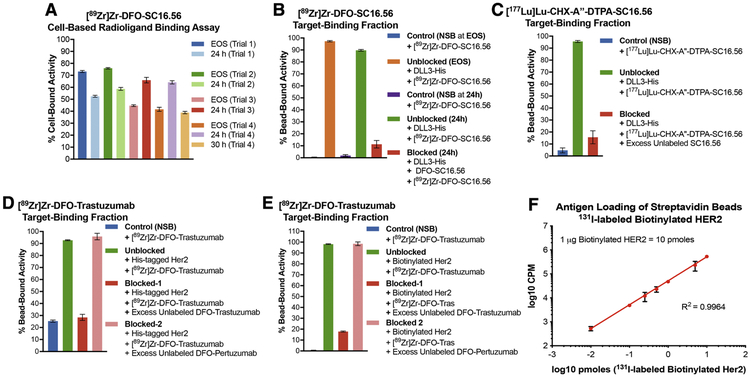
Figure 2. Applicability of bead-based radioligand binding assay for targets having low versus high cell-surface abundance.
A) Inconsistent results obtained from cell-based assays to determine the target-binding fraction (TBF) of [89Zr]Zr-DFO-SC16.56 (molar activity = 14 MBq/nmol) at end of synthesis (EOS) and 24 h after storage of the sample at −80 °C in 4 consecutive experimental trials done over a period of 4 weeks; B) Demonstration of high TBF for [89Zr]Zr-DFO-SC16.56 using Ni-NTA beads coated with His-tagged DLL3 and minimum non-specific binding (NSB) to naked Ni-NTA beads at (EOS) and 24 h later; C) High TBF demonstrated by [177Lu]Lu-CHX-A”-DTPA-SC16.56 (molar activity = 22.4 MBq/nmol) – a radioimmunotherapeutic variant of the DLL3-targeting antibody. Specificity of binding to antigen-coated beads for both the DLL3-targeting radioligands was validated in the presence of a 3500-fold excess of unlabeled SC16.56 immunoconjugates; D) Evaluation of the TBF of [89Zr]Zr-DFO-Trastuzumab (molar activity = 14 MBq/nmol) using Ni-NTA beads coated with His-tagged Her2 protein showed ~25% NSB of the radioligand to naked Ni-NTA beads; E) Using streptavidin beads coated with biotinylated Her2 to evaluate the TBF of [89Zr]Zr-DFO-Trastuzumab eliminated the NSB and demonstrated high specific binding, which could be blocked by excess unlabeled DFO-Trastuzumab, but not DFO-Pertuzumab; F) Using 131I-labeled biotinylated Her2 to determine the degree of loading of the streptavidin beads demonstrated linearity with increasing concentration of the antigen over at least 4 orders of magnitude. The error bars on each data point represent 95% CI. The plot validates that the bead-based assay is being operated in the pre-saturation concentration range when 1 μg (10 pmoles) of biotinylated Her2 was used with 20 μL (200 μg) of streptavidin beads.