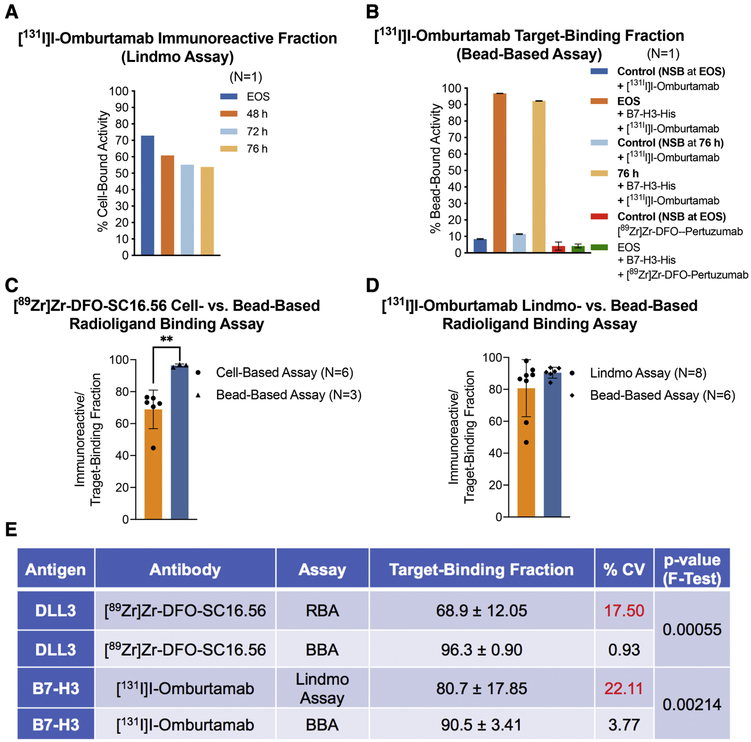
Figure 3. Comparative analysis of the performance of cell-based radioligand binding assay and the bead-based assay.
A) Poor and progressively decreasing immunoreactive fractions obtained from Lindmo assay of [131I]I-Omburtamab (molar activity = 392 MBq/nmol) with B7-H3-expressing LAN-1 cells at EOS, 48 h, 72 h and 76 h after radiosynthesis (radioligand samples/aliquots stored at −80 °C; B) High TBFs obtained for the same preparation of [131I]I-Omburtamab with His-tagged B7-H3 in a bead-based assay performed at EOS and 76 h after storage of the sample at −80 °C; C-D) Bar graphs showing a comparative analysis of the means from multiple experiments to evaluate the performance of cell-based radioligand binding assay (RBA) versus bead-based assay (BBA); E) Summarized statistics for the two methods of analyses showing superior intermediate precision obtained from use of BBA (%CV <5; F-test of equality of variances p-value <0.005).