Abstract
A 69-year-old woman was admitted to our hospital with a fever, dizziness, and headache caused by Neisseria meningitidis. After ceftriaxone was administered, she suddenly developed bilateral oculomotor nerve palsy. Intra-orbital magnetic resonance imaging using appropriate sequences revealed that her bilateral third intracranial nerves were enlarged and enhanced. She achieved complete recovery by two months after additional short-term treatment with intravenous immunoglobulin and methylprednisolone. Although intracranial nerve disorders that result from bacterial meningitis are most frequently reported in children, it is noteworthy that it can also cause focal intracranial nerve inflammation with ophthalmoparesis in N. meningitidis infection in adults.
Keywords: bilateral oculomotor nerve palsy, Neisseria meningitidis, magnetic resonance imaging
Introduction
Bacterial meningitis is an infectious disease of the central nervous system with an annual incidence of 4-6 cases per 100,000 adults (1,2). Neisseria meningitidis is the second-most common cause of community-acquired adult bacterial meningitis (3), and the disease can result in severe sequelae, which include cerebral lesions, hearing loss, learning difficulties and severe cognitive deficits, cerebral palsy, and epilepsy (4).
We herein report a rare adult case of N. meningitidis infection with acute bilateral oculomotor (third) nerve palsy. Other symptoms, however, were relatively mild for this type of infection, and the ocular symptoms were successfully treated using combined therapies of intravenous immunoglobulin and methylprednisolone in addition to antibiotic medication. In this case, the diagnosis was made based on the intra-orbital magnetic resonance imaging (MRI) findings and negative presence of antiganglioside antibodies.
Case Report
A 69-year-old Japanese woman presented to the emergency department with a fever (38.0℃), dizziness, and headache. She had no history of diabetes or glucose intolerance, hypertension, hypercholesterolemia, systemic vasculitis, smoking, or obesity.
A physical examination revealed that her vital signs were normal with a blood pressure of 132/72 mmHg, heart rate of 72 beats/min, and respiratory rate of 15 breaths/min. No rash or other abnormalities were noted on the skin of the entire body. A neurological examination found no motor laterality or pathological reflexes, except for nuchal rigidity. Her level of consciousness was normal. Laboratory screening revealed an increase in leukocytes (10,400 cells/μL) and a remarkable increase in the C-reactive protein (CRP) level (25.20 mg/dL). Blood coagulation tests showed significantly increased fibrin/fibrinogen degradation products (FDP) (>300 mg/dL, normal <5) and D-dimer levels (>100 μg/mL, normal <1). Based on the blood examination results, diagnostic lumbar puncture was performed, and turbid yellow cerebrospinal (CSF) fluid was collected with an opening pressure of 17 mmHg (normal 8-15 mmHg). An examination of the CSF revealed a cell count of 28 cells/mm3 (polymorphonuclear leukocytes: 19 cells/mm3; mononuclear leukocytes: 9 cells/mm3), protein level of 468 mg/dL, and glucose level of 2 mg/dL, with a plasma glucose level of 155 mg/dL; all values were within normal reference ranges. The diagnosis of bacterial meningitis was made, and the patient was immediately started on antimicrobial therapy (ceftriaxone 4 g/daily). A blood culture analysis the next day revealed the presence of N. meningitidis.
The day after ceftriaxone was administered (second hospital day), the patient suddenly exhibited bilateral ptosis and eye deviation. A neurological examination revealed bilateral oculomotor nerve palsy that was particularly severe on the right side (Fig. 1A). The pupils on both sides were 4.0 mm in diameter, exhibiting mydriasis with a sluggish light reflex. All other cranial nerves were intact. MRI (1.5T Signa HDxt, GE Healthcare, Waukeshau, USA) on the third hospital day revealed significant high-signal intensities of oculomotor nerves bilaterally on fat-suppressed T2-weighted images (Fig. 2A), fat-suppressed gadolinium-enhanced T1-weighted images in the intra-orbital coronal plane (Fig. 2B), and in the axial plane (Fig. 2C). Magnetic resonance angiography did not reveal any abnormalities in the orbits or the brain. Bilateral oculomotor nerve palsies were persistent under monotherapy with ceftriaxone, although the fever and headache resolved. Therefore, on day 10, both intravenous immunoglobulin (0.4 g/kg bodyweight for 5 days) and methylprednisolone (1,000 mg/daily for 3 days and continuously 500 mg/daily for 2 days) were added to the therapy. The levels of antiganglioside immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in the patient's serum on day 10 were measured by a semi-quantitative enzyme-linked immunosorbent assay. No IgG and IgM antibodies against GM1, GM2, GM1b, GD1a, GD1b, GT1a, GQ1b, or GalNAc-GD1a were detected. No indicators of autoimmune disease, including serum anti-acetylcholine receptor and IgG4 antibodies, myelin basic protein, and oligoclonal IgG bands in the CSF, were observed. Intracranial MRI showed no abnormalities apart from bilaterally enlarged oculomotor nerves. Thus, a diagnosis of N. meningitidis infection and acute ophthalmoparesis was made.
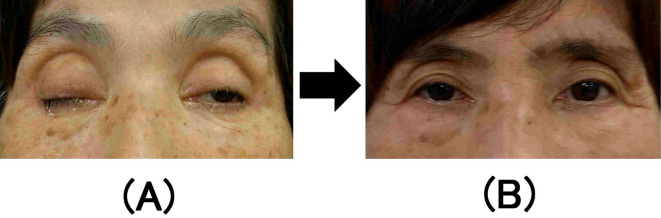
Figure 1.
(A) Bilateral oculomotor nerve palsy on day 3, more severe on the right side than on the left. (B) The patient fully recovered from bilateral oculomotor nerve palsy after two months. The patient provided her informed consent to use the medical photographs for publication purposes.
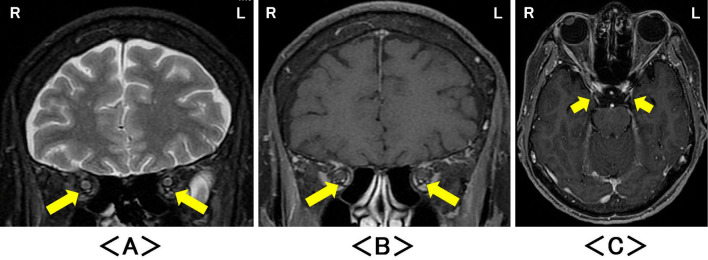
Figure 2.
Magnetic resonance imaging after the onset of oculomotor nerve palsy revealed significant high-signal intensities of oculomotor nerves bilaterally on (A) coronal intra-orbital fat-suppressed T2-weighted images (arrow), and significant enlargement and the enhancement on (B) coronal intra-orbital and (C) axial fat-suppressed T1-weighted images with gadolinium enhancement (arrow). R: right, L: left
The patient's ocular symptoms improved gradually after the administration of intravenous immunoglobulin and methylprednisolone, and antibiotic medication with ceftriaxone was continued for three weeks. On the 27th hospital day, a follow-up examination of the CSF showed that it was clear, and further testing revealed a cell count of 45 cells/mm3 (polymorphonuclear leukocytes: 44 cells/mm3; mononuclear leukocytes: 1 cell/mm3), a protein level of 77.2 mg/dL, and a glucose level of 52 mg/dL, with a plasma glucose level of 106 mg/dL. At discharge on Day 35, blood examination results were within the normal range: white blood cells 4,500 cells/μL and CRP 0.66 mg/dL. Complete recovery from ophthalmoparesis on both sides was observed after two months (Fig. 1B) without any other additional immunosuppressive medication. Follow-up MRI showed no abnormalities of the oculomotor nerves on fat-suppressed T2-weighted or gadolinium-enhanced T1-weighted images (Fig. 3).
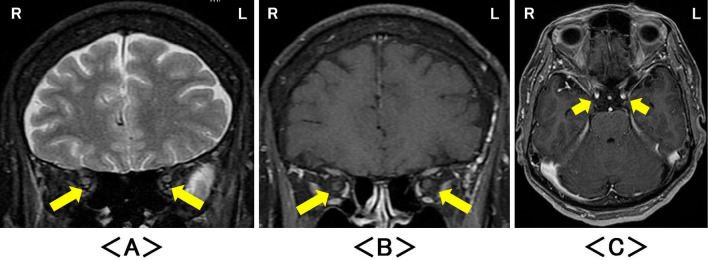
Figure 3.
Magnetic resonance imaging two months later revealed no abnormalities in either oculomotor nerve on (A) coronal intra-orbital fat-suppressed T2-weighted images (arrow), (B) coronal intra-orbital and (C) axial fat-suppressed T1-weighted images with gadolinium enhancement (arrow). R: right, L: left
Discussion
This report describes a case involving an adult patient with N. meningitidis infection and acute bilateral oculomotor nerve palsy that was identified based on MRI findings. Oculomotor nerve palsy can be caused by several disorders, including cerebral aneurysms, vascular disorders, tumors, and diabetes mellitus. However, in this case, no clinical, laboratory, or imaging examinations showed any indication of an underlying structural cause of the oculomotor nerve injury, suggesting that N. meningitidis infection might have caused the transient oculomotor nerve palsy in this case.
Intracranial nerve disorders caused by N. meningitidis infection, including oculomotor nerve and abducens (sixth) nerve palsies, are extremely rare, and to our knowledge, only one case has been reported (5). The oculomotor nerve palsy in the reported case was suspected to be caused by local ischemia accompanied by nerve dysfunction. However, intra-orbital MRI was not performed (5).
In the present case, intra-orbital MRI (coronal plane) at the acute stage revealed significantly enlarged and enhanced signals in the oculomotor nerves on fat-suppressed T2-weighted and gadolinium-enhanced images. Some of the major causes of third nerve palsy include aneurysms of the posterior communicating artery, microvascular ischemia, neoplasm, inflammation, and trauma (6). Microvascular ischemia, which is often associated with diabetes mellitus and hypertension, is believed to be the most common cause of third nerve palsies, and compression by aneurysms or tumors can also lead to pupil-sparing oculomotor nerve palsy (7). Lumbar puncture with overdrainage of the CSF that leads to intracranial hypotension with descent of the brain, has also been reported to cause traction of the cranial nerves (8). However, the current case was not a result of these factors.
Our previous case report described influenza A infection causing acute isolated unilateral oculomotor nerve palsy presenting as focal intracranial nerve inflammation with ophthalmoparesis and spontaneous recovery (9). These disorders were previously able to be diagnosed through intra-orbital MRI with appropriate sequences and immunological assays to detect the presence of antiganglioside antibodies (10). However, no abnormalities of antiganglioside antibodies were observed in the present case.
The present case is also the first report of an adult patient with bilateral oculomotor nerve palsy presenting as ophthalmoparesis, which was identified based on intra-orbital MRI. Although neural disorders arising after N. meningitidis infection, including intracranial vascular involvements with ischemic infarction, sinus thrombosis, and hemorrhagic complications, are most frequently reported (11), it is important to note that N. meningitidis infections can also cause focal neurological signs, such as ophthalmoparesis, as observed in this case. Intra-orbital MRI with adequate sequences, such as fat suppression sequences, are more suitable for detecting the presence of these disorders than conventional MRI (12,13). Furthermore, examinations for the presence of antiganglioside antibodies can be performed to support pathology, as in the present case.
Once N. meningitidis reaches the CSF, it replicates rapidly due to the absence of host immune defense mechanisms in this microenvironment. The cell wall of N. meningitidis contains lipooligosaccharides (LOSs), which have a strong endotoxin activity. The LOS levels in both the plasma and CSF are significantly correlated with the disease prognosis (14). In the present case, we speculate that cytokine storm caused by the LOS might have occurred at the patient's initial visit, thus resulting in the discrepant results of low cell counts and high protein levels in the CSF at her first examination.
Why focal neurological deficits may arise following N. meningitidis infections remains unclear; pathological mechanisms, such as focal vasculitis via direct bacterial invasion or inflammation accompanying the effects of antibiotics, are commonly suggested (15,16). However, tumors, including orbital schwannoma, demyelinating diseases, as well as damage to the myelin sheaths and surrounding axons due to complications cannot be completely excluded (17,18). Additional testing of the CSF, which we were unable to perform, may be needed to confirm the presence of N. meningitidis, such as bacterial antigen detection tests for N. meningitidis. It is still controversial why a full recovery from bilateral oculomotor nerve palsy was achieved with immunosuppressive therapy consisting of immunoglobulins and corticosteroids, which is considered to be an appropriate treatment for inflammatory neuropathy and related diseases (19). It is possible that the same symptoms or signs may be observed with other types of bacterial meningitis. Thus, further investigations are required to determine the appropriate treatment strategy for cases such as the one described in the present report.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
The authors state that they have no Conflict of Interest (COI).
Joe Senda and Takeshi Adachi contributed equally to this work.
Acknowledgement
The authors extend their gratitude to Dr. Susumu Kusunoki, Department of Neurology, Kindai University Faculty of Medicine, for the measurement of the anti-ganglioside antibodies.
References
- 1. Sigurdardottir B, Bjornsson OM, Jonsdottir KE, Erlendsdottir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 157: 425-430, 1997. [DOI] [PubMed] [Google Scholar]
- 2. Hussein AS, Shafran SD. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore) 79: 360-368, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Durand ML, Calderwood SB, Weber DJ, et al. . Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 328: 21-28, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Heckenberg SG, de Gans J, Brouwer MC, et al. . Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore) 87: 185-192, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Chiu CH, Lin TY, Huang YC. Cranial nerve palsies and cerebral infarction in a young infant with meningococcal meningitis. Scand J Infect Dis 27: 75-76, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol 27: 257-268, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Jacobson DM. Relative pupil sparing third nerve palsy: etiology and clinical variables predictive of a mass. Neurology 56: 797-798, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Vakharia SB, Thomas PS, Rosenbaum AE, Wasenko JJ, Fellows DG. Magnetic resonance imaging of cerebrospinal fluid leak and tamponade effect of blood patch in postdural puncture headache. Anesth Analg 84: 585-590, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Senda J, Araki K, Tachi Y, et al. . Acute unilateral isolated oculomotor nerve palsy in an adult patient with influenza A. Intern Med 58: 433-436, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuki N, Odaka M, Hirata K. Acute ophthalmoparesis (without ataxia) associated with anti-GQ1b IgG antibody: clinical features. Ophthalmology 108: 196-200, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Bodilsen J, Dalager-Pedersen M, Schonheyder HC, Nielsen H. Stroke in community-acquired bacterial meningitis: a Danish population-based study. Int J Infect Dis 20: 18-22, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Tien RD, Chu PK, Hesselink JR, Szumowski J. Intra- and paraorbital lesions: value of fat-suppression MR imaging with paramagnetic contrast enhancement. AJNR Am J Neuroradiol 12: 245-253, 1991. [PMC free article] [PubMed] [Google Scholar]
- 13. Ettl A, Kramer J, Daxer A, Koornneef L. High resolution magnetic resonance imaging of neurovascular orbital anatomy. Ophthalmology 104: 869-877, 1997. [DOI] [PubMed] [Google Scholar]
- 14. Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369: 2196-2210, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Lesourd A, Magne N, Soares A, et al. . Primary bacterial ventriculitis in adults, an emergent diagnosis challenge: report of a meningoccal case and review of the literature. BMC Infect Dis 18: 226, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain S, Win HN, Chalam V, Yee L. Disseminated gonococcal infection presenting as vasculitis: a case report. J Clin Pathol 60: 90-91, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tantiwongkosi B, Hesselink JR. Imaging of ocular motor pathway. Neuroimaging Clin N Am 25: 425-438, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Keane JR. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci 37: 662-670, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Eldar AH, Chapman J. Guillain Barré syndrome and other immune mediated neuropathies: diagnosis and classification. Autoimmun Rev 13: 525-530, 2014. [DOI] [PubMed] [Google Scholar]