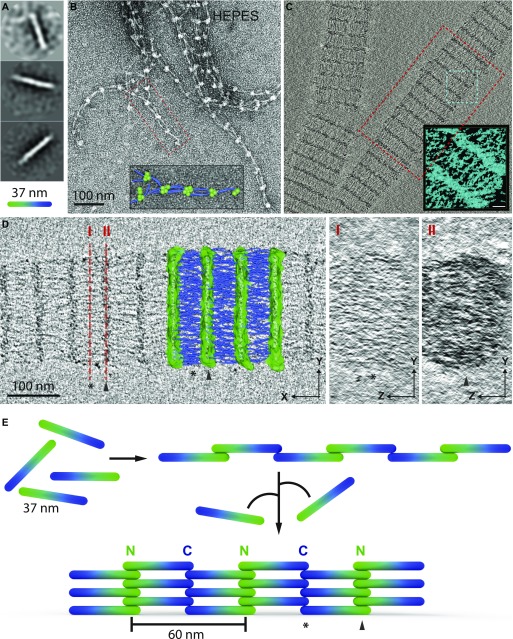
Figure 6. Intermediate stages of FilP filament assembly and a model of bundle formation.
(A) Single-particle 2D class averages from negative staining images of FilP dialyzed in 20 mM ethanolamine, pH 11, buffer, which shows 37 nm rods representing the primary state of filament assembly. Particle images are cropped and rotated for 2D classification, the class averages are consequently displayed as circular images on an average grey background. (B) Negative staining EM of FilP dialyzed in Hepes buffer display FilP in the form of individual protofilaments and thin bundles. “Bead on a string” structures are modeled as a head-to-head and tail-to-tail association of FilP rods, resembling protofilaments in the selected magnified box. Green is used to highlight the densities of the N-terminal His-tag, and blue color is used for the rod coiled-coil, including the C-terminal portion of the protein. (C) Tomogram central slice of FilP filament bundles/paracrystals show protofilaments in longitudinal, branching, and lateral association. In the selected turquoise box, a subvolume is visualized by IMOD isosurface rendering (scale bar = 20 nm). (D) Tomogram selected red box from (C) showing both a projection of 20 pixels in Z from the center of the volume and a model, drawn in 3Dmod, of tomogram protein densities. Green mesh represents the densities of the protein-dense major bands (containing the N-terminus of FilP) and blue rods represent the protofilament transversally bridging between bands. Along I and II, the tomogram volume was rotated 90° around Y to show a projection in X of the major band (I) and the minor band (II) volumes, lacking apparent structural pattern or crystal packing. (E) Model of FilP assembly: 37 nm rod domains proposed to consist of FilP parallel coiled-coil dimers assembled into protofilaments. Lateral association of additional primary assembly units results in thick filament bundles and protofilaments with a 60 nm repetitive striation pattern.