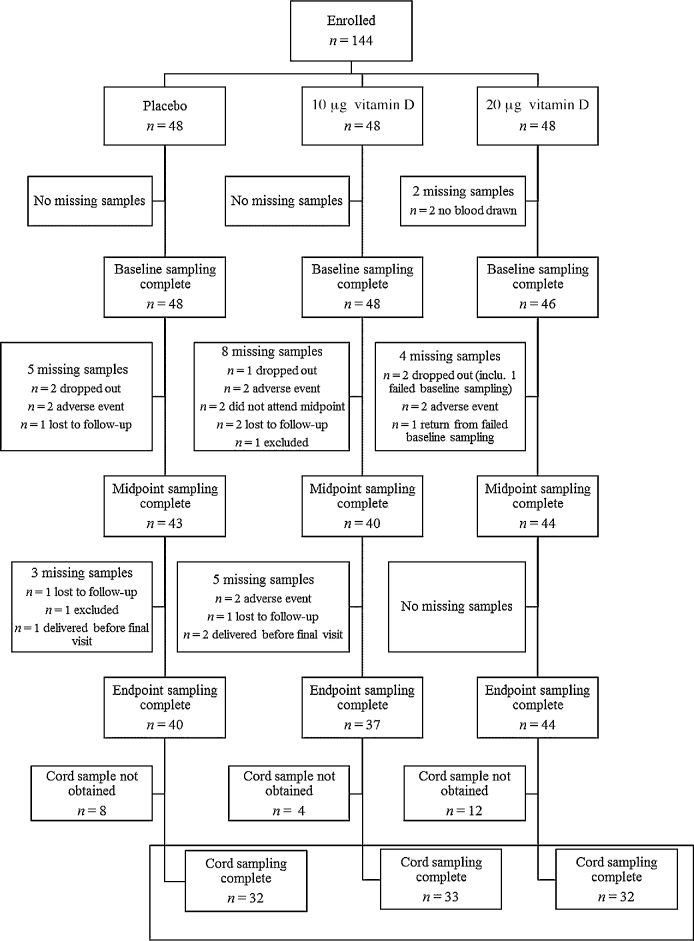
FIGURE 1.
CONSORT flow diagram of participant enrollment, random assignment, and biochemical analysis throughout the study by treatment group, where n is based on the total number of samples available for each visit. Any participant who did not provide a blood sample at baseline was included in the descriptive and biochemical analysis at later time points but excluded from the dose-response analysis, whereas women missing a midpoint sample only were included in both the dose-response analysis and the analysis at endpoint, if a blood sample was collected at this time point. The numbers of women who provided both a baseline and ≥1 follow-up sample (midpoint or endpoint) were 43, 42, and 43 for the placebo and 10- and 20-µg groups, respectively, which left a final number of 128 for the dose-response analysis. CONSORT, Consolidated Standards of Reporting Trials; inclu., including.