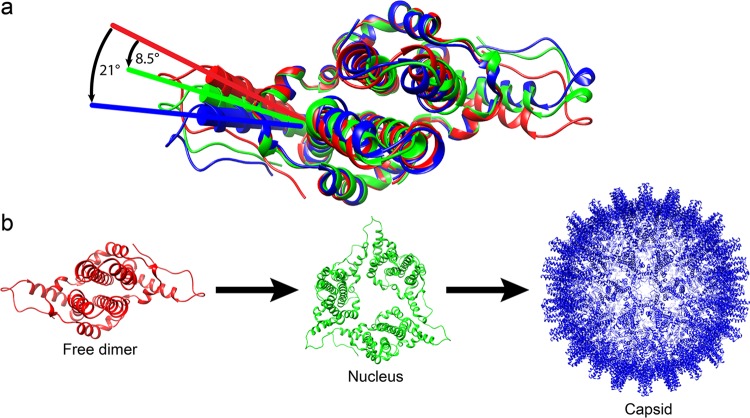
FIG 8.
α-Helix 5 modulates the capsid assembly process by changing its conformations. (a) Alignment of wY132A (red), hY132A (green; PDB accession number 3KXS), and dimer in WHV capsid (blue) shows that the three dimers have different positions for residues from α-helix 5 to the C termini. wY132A needs to shift 8.5° to match the conformation of hY132A and 21° to match wCp149. (b) A hypothesized scheme of the assembly process that progresses through the different dimer conformations (structures are labeled using the same colors used in panel a). We propose the following sequence of events. Assembly starts with free dimers (wY132A; red). Initiation of assembly induces a 8.5° shift of α-helix 5 to generate nuclei (based on hY132A, trimer of dimers) (11, 13). As assembly proceeds, the α-helix of dimers keep rotating to 21°, where dimers have a conformation that corresponds to those in a capsid (WHV capsid).