Table 3.
The conversion and enantiomeric excess results of a sequential process reaction combining the use of a ruthenium-catalyzed isomerization and a bioreduction in the presence of DES [40].
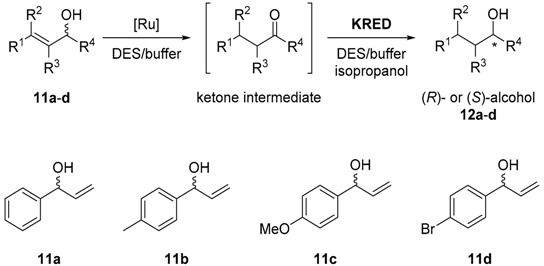
| Entry | Substrate | Product | Solvent | ADH | Conversion (%)(c) | ee (%) |
|---|---|---|---|---|---|---|
| 1 (a) | 11a | 12a | ChCl:Gly (1:2 mol/mol, 50% w/w) | P1-A04 | >99 (95) | >99 (R) |
| 2 (a) | 11a | 12a | ChCl:Gly (1:2 mol/mol, 50% w/w) | L. kefiri | >99 (92) | >99 (R) |
| 3 (a) | 11b | 12b | ChCl:Gly (1:2 mol/mol, 50% w/w) | P2-H07 | 95 (90) | >99 (R) |
| 4 (a) | 11b | 12b | ChCl:Gly (1:2 mol/mol, 50% w/w) | L. kefiri | 70 (64) | 93 (R) |
| 5 (a) | 11c | 12c | ChCl:Gly (1:2 mol/mol, 50% w/w) | P3-H12 | 95 (90) | >99 (S) |
| 6 (a) | 11c | 12c | ChCl:Gly (1:2 mol/mol, 50% w/w) | L. kefiri | 65 (60) | 93 (R) |
| 7 (a) | 11d | 12d | ChCl:Gly (1:2 mol/mol, 50% w/w) | P2-H07 | 94 (90) | >99 (R) |
| 8 (a) | 11d | 12d | ChCl:Gly (1:2 mol/mol, 50% w/w) | L. kefiri | 80 (72) | 98 (R) |
| 9 (b) | 11a | 12a | ChCl:Gly (1:2 mol/mol, 80% w/w) | P2-C11 | 90 | >99 (R) |
| 10 (b) | 11b | 12b | ChCl:Gly (1:2 mol/mol, 80% w/w) | P2-C11 | 70 | >99 (R) |
| 11 (b) | 11c | 12c | ChCl:Gly (1:2 mol/mol, 80% w/w) | P2-C11 | 68 | >99 (R) |
| 12 (b) | 11d | 12d | ChCl:Gly (1:2 mol/mol, 80% w/w) | P2-C11 | 96 | >99 (R) |
| 13 (b) | 11a | 12a | ChCl:Gly (1:2 mol/mol, 80% w/w) | L. kefiri | 21 | >99 (R) |
(a) Reaction performed in a sequential mode. (b) Reaction performed in a concurrent manner. (c) Isolated yields appear in brackets.
