Abstract
The management of advanced gastric cancer has improved over the past decade. There is more evidence to support the efficacy of systemic treatment in refractory gastric cancer beyond second-line treatment. Important randomized controlled trials of chemotherapies, targeted agents and immunotherapies have been reported. With the development of these novel therapies, clinicians can better individualize treatment for patients beyond progression on second-line therapy. However, there is no guideline on third-line therapy available for clinicians. This review discussed the efficacy and safety data from the pivotal trials of the agents proven to be effective in third-line settings, including the quality of study design, level of evidence and subgroup analysis, and how the data can help to guide clinicians on selecting the most appropriate third-line therapy for their patients.
Keywords: gastric cancer, immunotherapy, metastatic, palliative, systemic treatment
Introduction
Gastric cancer is the fifth commonest cancer and remains the world’s third leading cause of cancer mortality.1 Surgery is the mainstay of curative treatment in stage I to III gastric cancers. However, more than half of the patients at diagnosis are already too advanced for curative resection. Even for those who are resectable upfront, the recurrence rate is still high at around 40–80%.2,3
First-line then second-line palliative chemotherapy is the standard of treatment in patients with advanced/metastatic gastric cancer. A Cochrane review and meta-analysis performed by Wagner demonstrated that chemotherapy extended overall survival (OS) by approximately 6.7 months more than best supportive care [BSC; hazard ratio (HR) 0.3; 95% confidence interval (CI) 0.24–0.55, p < 0.0001].4 Standard front-line therapy includes chemotherapy using fluorouracil (5FU) and platinum agents, with the option of adding anthracycline or taxane group agents. In human epidermal growth factor receptor 2 (Her-2) positive advanced gastric cancer, as proven in the TOGA study, adding trastuzumab to platinum-based chemotherapy (cisplatin/carboplatin + 5FU) showed superior efficacy compared with chemotherapy alone (OS: 13.8 versus 11.1 months, HR 0.74; 95% CI 0.60–0.91; p = 0.0046).5
Several systematic reviews and meta-analyses had confirmed survival advantage of second-line chemotherapy when compared with BSC alone.6–8 In Kim and colleagues’ meta-analysis, which involved 410 patients, second-line chemotherapy significantly reduced the risk of death when compared with BSC (HR 0.64, 95% CI 0.52–0.79, p < 0.0001).6 Standard second-line therapies include irinotecan-based and taxane-based (docetaxel or paclitaxel) chemotherapy. Ramucirumab, a vascular endothelial growth factor receptor (VEGFR) monoclonal antibody, has also been established as monotherapy or in combination with paclitaxel in the second-line setting. Several network meta-analyses have been published to compare these second-line regimes. Combination of paclitaxel plus ramucirumab showed superior efficacy in prolonging OS when compared with single-agent chemotherapy or ramucirumab.9–11
With the development of new chemotherapies or targeted agents which are potentially more effective and less toxic, many patients can still maintain a good general condition after failing second-line therapies. According to previous studies, around 20–90% patients were able to continue on active third-line or further lines of treatment.
Established third-line therapies include chemotherapies: irinotecan, taxane and TAS-102, tyrosine kinase inhibitors: apatinib and regorafenib, and immune-checkpoint inhibitors (CPIs): nivolumab and pembrolizumab. Given the expanding options for third-line therapies, there is an unmet need for clinicians to individualize treatment. In this review, we discuss the efficacy and safety results from the pivotal trials of the proven third-line therapies, including the study design, level of evidence, subgroup analysis and formulate comprehensive strategies to guide clinicians to select the most appropriate treatment for individual patients.
Materials and methods
Electronic databases with MEDLINE and EMBASE Ovid were searched for the pivotal trial publications. Handsearching for the published abstracts and presentations at conferences was performed: The American Society for Clinical Oncology 2000 to 2018 and The European Society for Medical Oncology 2000 to 2018 (published in the Annals of Oncology). The prescribing information for each agent was reviewed.
The real-world practice on the use of third-line treatment
Data are now emerging to support treatment in the third-line treatment setting. In a large retrospective South Korean study with 1435 patients, 27% of the patients with advanced gastric cancer were treated with systemic third-line treatment.12 Another analysis of a national health insurance database found that 21% of patients with advanced gastric cancer received third-line chemotherapy and the median OS was 4.4 months.13 The OS was associated with the progression-free survival (PFS) from first-line chemotherapy to third-line chemotherapy.
The use of systemic treatment beyond the second-line is less common but is increasingly adopted in western countries. A retrospective review of 511 cases treated in the Royal Marsden Hospital in the United Kingdom from April 2009 to November 2015 reported that 71 patients (14%) received third-line treatment. Of these 71 patients, 2 (3%), 26 (37%), and 42 (60%) received triplet-, doublet-, or single-agent therapy, respectively.14 In another multicenter, retrospective study involving 2200 patients with advanced gastric cancer treated from May 2000 to February 2015 in 19 Italian oncology departments, 331 patients (15.0%) received a third-line therapy.15 A total of 45.7% of patients received single-agent chemotherapy while 49.7% received a combo regimen. Patients who achieved a first-line PFS ⩾ 6.9 months or a second-line PFS ⩾ 3.5 months had better prognosis compared with those who did not.
Overall efficacy from meta-analysis
A systematic review and meta-analysis on third-line systemic treatment involving six randomized controlled trials (RCTs) and 890 participants, of which 76.2% were Asian, showed that third-line treatment improved OS (HR 0.63; 95% CI 0.46–0.87, corresponding to an improvement in median OS from 3.20 to 4.80 months) and PFS (HR 0.29; 95% CI 0.18–0.45) when compared with BSC.16 The HR for median survival in Asian populations was 0.63 (95% CI 0.45–0.90, p = 0.01) in favor of third-line treatment, corresponding to an improvement in median OS from 3.20 months to 4.83 months. The magnitude in OS benefit in the Asian subgroup was similar to the whole population.
These evidences implied that a proportion of patients could tolerate and gained benefit from a sequenced treatment approach incorporating multiple lines of therapy in both the East and the West.
Agents with proven efficacy in the third-line setting
Table 1 shows the summary efficacy data from the pivotal studies.
Table 1.
Agents proven to be effective in the third-line setting in advanced gastric cancer.
| Third-line treatment agent | Study | Phase | Interventions | Number of participants | Setting | Inclusion criteria | Exclusion criteria | Country | Overall survival | Progression-free survival | Overall response rate | Disease control rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irinotecan/ taxane17 | NCT00821990 | III | Irinotecan 150 mg/m2 i.v. every 2 weeks or docetaxel 60 mg/m2 i.v. every 3 weeks versus placebo | 202 (third-line only: 54) |
Second-and third-line | - Received one or two prior chemotherapy involving both
platinum and 5FU - ECOG 0/1 |
- More than two prior chemotherapy regimens - Prior exposure to both taxanes and irinotecan - Significant comorbidities |
Korea | 5.3 versus 3.8 months
(p = 0.007) Third-line only: HR 0.812, p = 0.173 |
|||
| TAS-10218 | TAGS (NCT02500043) |
III | TAS-102 35 mg/m2 twice daily p.o. on D1–5 & D8–12 of 28-day cycle versus placebo | 507 | Third or further line | - Received two or more chemotherapies for advanced disease
involving platinum, 5FU, taxane or irinotecan
- Her-2 +ve: must have received anti-Her-2 therapies |
- CNS metastasis - Active infection - Serious organ dysfunction - Autoimmune disorders - Uncontrolled diabetes - GI hemorrhage within 2 weeks - History of organ transplantation requiring immunosuppressive therapy |
Multinational a | 5.7 versus 3.6 months (p = 0.0003) | 2.0 versus 1.8 months (p < 0.0001) | 4% versus 2% (p = 0.28) | 44% versus 14% (p < 0.0001) |
| Apatinib19 | NCT01512745 | III | Apatinib 850 mg p.o. daily versus placebo | 267 | Third-line | - Received two or more lines of chemotherapy - ECOG 0 or 1 |
- Uncontrolled hypertension - Bleeding tendency - Receiving thrombolytics or anticoagulants - Prior VEGFR inhibitors - Ascites required interventions |
China | 6.5 versus 4.7 months (p = 0.0149) | 2.6 versus 1.8 months (p < 0.001) | 2.84% versus 0% (p = 0.17) | 42.05% versus 8.79 % (p < 0.001) |
| Regorafenib20 | INTEGRATE | II | Regorafenib 80–120 mg p.o. daily D1–21 of 28-day cycle versus placebo | 147 (third-line only: 85) |
Second-and third-line | - Received one or two lines of chemotherapy involving 5FU
and platinum - Adequate renal (CrCl >50 ml/min) and liver function - ECOG 0 or 1 |
- Uncontrolled hypertension - Uncontrolled CNS disease - Prior anti-VEGF therapy - Known malabsorption syndrome - Arterial thromboembolic events (e.g. cerebral vascular accident or pulmonary embolism within 6 months) - Venous thromboembolic events within 3 months |
Australia, New Zealand, Canada, South Korea | 5.8 versus 4.5 months
(p = 0.147) Third-line only: HR 0.22, p < 0.001 |
2.6 versus 0.9 month (p < 0.001) | 3.09% versus 0.02% | 43.3% versus 16% |
| Nivolumab21 | ATTRACTION-2 ONO-4538-12 (NCT02267343) |
III | Nivolumab 3 mg/kg i.v. every 2 weeks versus placebo | 493 | Third or further line | - Received two or more lines of chemotherapy - ECOG 0 or 1 |
- Ongoing or previous autoimmune disease or interstitial
lung disease - Active diverticulitis or GI ulcerative disease - Brain metastasis which were symptomatic or required treatment - Significant medical disorders - Prior anti-PD-1 therapy or other therapies that regulate T-cells |
Japan, South Korea, Taiwan | 5.26 versus 4.14 months (p < 0.0001) | 1.61 versus 1.45 months (p < 0.0001) | 11.2% versus 0% | 40% versus 25% |
| Pembrolizumab22 | KEYNOTE-059 | II | Pembrolizumab 200 mg i.v. every 3 weeks | 259 | Third or further line | - Received two or more lines of chemotherapy involving 5FU
and platinum - Her-2 +ve: must have received anti-Her-2 therapies - ECOG 0 or 1 |
- Clinical evidence of ascites - Active immune disease that required systemic treatment in the preceding 2 years - Immunodeficiency - Active noninfectious pneumonitis - Receiving systemic steroid or any immunosuppressive therapy - Prior anticancer monoclonal antibodies or targeted small-molecule or radiotherapy within 4 weeks before study - Prior immune-checkpoint blocking therapy - Known CNS metastasis - Hepatitis B/C |
Multinationalb | 5.6 months | 2.0 months | Third-line: 16.4% Fourth-line: 6.4% PD-L1 +ve: 15.5% (CR: 2%) PD-L1 –ve: 6.4% (CR: 2.8%) MSI-H: 57.1% |
16.2% |
5FU, fluorouracil; CNS, central nervous system; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; Her-2, human epidermal growth factor receptor; HR, hazard ratio; i.v., intravenous; MSI-H, microsatellite instable high; NCT, ClinicalTrials.gov identifier; PD-1, programmed cell death 1; PD-L1, programmed death ligand 1; p.o., per oral; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Belarus, Belgium, Canada, Czechia, France, Germany, Ireland, Israel, Italy, Japan, Poland, Portugal, Romania, Russian Federation, Spain, Turkey, United Kingdom, United States.
Australia, Canada, Chile, Colombia, Estonia, France, Israel, Italy, Japan, Korea, Republic of, Lithuania, Peru, Portugal, Romania, Russian Federation, United Kingdom, United States.
Chemotherapy
Taxane or irinotecan
Both taxane (paclitaxel/docetaxel) and irinotecan, as monotherapy or in combination with cisplatin/ 5FU, are commonly used as second- or third-line settings in patients with advanced gastric cancer. In a multicenter, phase III Korean RCT reported by Kang and colleagues, 202 patients with advanced gastric cancer with one or two prior chemotherapy regimens involving both platinum and 5FU and with an Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1 were randomized into salvage chemotherapy using docetaxel or irinotecan versus BSC alone. Salvage chemotherapy significantly improved the OS versus BSC (5.3 versus 3.8 months, HR 0.657, p = 0.007) with a 34% reduction in the hazard of death.17 The study did not report the PFS or response rate. However, in the subgroup analysis, the OS benefit was not significant in patients with two prior lines of treatment (n = 54, HR 0.812, 95% CI 0.450–1.464, p = 0.173).
The commonest treatment-related adverse events (TRAEs) of any grade in the chemotherapy arm were myelosuppression: neutropenia (60.3%), anemia (76.2%), thrombocytopenia (23.0%), and fatigue (45.1%). The most common G3/4 TRAEs included anemia (29.3%), neutropenia (15.8%), fatigue (17.3%), anorexia (5.3%), and diarrhea (5.3%).
More patients in the chemotherapy arm continued for further lines of active treatment compared with the BSC arm (40% versus 22%, p = 0.011) and the median OS was longer for patients who received subsequent therapy than those who did not (median OS 8.0 versus 3.7 months; p < 0.001).
TAS-102
TAS-102 (Taiho Pharmaceutical, Tokyo, Japan) is an oral combination drug of two active compounds: trifluridine, a thymidine analog (nucleoside antitumor agent), and tipiracil hydrochloride, a thymidine phosphorylase inhibitor, in a ratio of 1:0.5. In the international phase III RCT TAGS study carried out in 110 academic hospitals in 17 countries, 507 advanced/metastatic gastric adenocarcinomas (including adenocarcinoma of the gastroesophageal junction) patients were randomized to TAS-102 or placebo in a 2:1 ratio.18 Eligible patients should have previously received two or more previous chemotherapies for advanced disease and had experienced radiological disease progression within 3 months of the last dose of the previous treatment. Previous regiments must have included 5FU, a platinum agent, and taxane or irinotecan or both. Patients with Her-2 positive tumors must have received previous anti-Her-2 therapy. TAS-102 demonstrated a significant improvement in OS, 31% improvement relative to placebo (median OS: 5.7 versus 3.6 months, p = 0.0058). It also improved the PFS with a relative 43% improvement (PFS: 2.0 versus 1.8 months, HR 0.57, p < 0.0001). More patients in the TAS-102 arm achieved disease control compared with the placebo group (44% versus 14%, p < 0.0001).
Grade ⩾3 adverse events occurred in 80% of patients receiving TAS-102 versus 57.7% patients receiving placebo. The most common Grade ⩾3 adverse events (AEs) included neutropenia (38%), anemia (19%), leukopenia (9%), decreased appetite (9%) and fatigue (7%). Although there was a high rate of Grade ⩾3 neutropenia, febrile neutropenia was reported in six patients (2%) in the TAS-102 group. Dose reduction was more frequent with TAS-102 than with placebo (58% versus 22%). Overall, 13% patients in the TAS-102 group had AE-related treatment discontinuation, with most frequently reported reasons including general deterioration in physical health (n = 4, 1%), and thrombocytopenia (n = 3, 1%).
Targeted agents
1. Apatinib
Apatinib is an orally bioavailable, small-molecule tyrosine kinase inhibitor that highly selectively binds to and strongly inhibits VEGFR-2. In the pivotal phase III RCT conducted in China, 267 histologically confirmed advanced or metastatic adenocarcinomas of stomach patients, who failed with second-line chemotherapy with ECOG 0 or 1, were randomized to apatinib (850 mg daily orally) and placebo.19 Patients with uncontrolled blood pressure with medication (>140/90 mmHg), those with a bleeding tendency, and those receiving thrombolytics or anticoagulants were not eligible for this trial. Apatinib showed significant clinical benefits compared with placebo in terms of OS (6.5 versus 4.7 months, HR 0.709, p = 0.0156) and PFS (2.6 versus 1.8 months, HR 0.444, p < 0.001). The major benefit of apatinib was disease stabilization at a rate of 42.05% versus 8.79% for placebo. In subgroup analysis of OS, the extent of the OS benefit was notable for patients with fewer than two metastatic sites (HR 0.7, 95% CI 0.51–0.97).
The commonest nonhematologic AEs were proteinuria (47.7%), hypertension (35.2%), and hand-foot syndrome (27.8%). G3/4 toxicities with an incidence of ⩾5% of participants included hand-foot syndrome (8.5%), liver toxicities with elevation of bilirubin (7.4%), alanine aminotransferase (ALT; 8.0%), gamma-glutamyl transpeptidase (GGT; 6.3%), and hematological toxicities with neutropenia (5.7%) and anemia (6.3%). Based on this phase III study result, apatinib was approved in October 2014 by the China Food and Drug Administration for metastatic gastric or gastroesophageal junction adenocarcinoma after second-line chemotherapy. A global phase III study on apatinib is now ongoing to confirm its efficacy and generalizability in western patients.
2. Regorafenib
Regorafenib is an oral multi-kinase inhibitor, which inhibits angiogenesis [epidermal growth factor receptor (EGFR)1, 2, and 3; TIE2; platelet-derived growth factor receptor (PDGFR)-alpha and beta; and fibroblast growth factor receptor (FGFR)1 and 2], cancer-associated fibroblast-induced metastasis (PDGFR), and oncogenesis (RAF, RET, and KIT). In the phase II RCT INTEGRATE study, 147 recurrent or metastatic gastric cancer (gastroesophageal junction or stomach, adenocarcinoma, or undifferentiated histology) patients who were refractory to one or two lines of chemotherapy (including prior 5FU and platinum) were randomized to oral regorafenib and placebo.20 Patients with poorly controlled hypertension, prior anti-VEGFR therapy, and uncontrolled central nervous system (CNS) disease were excluded. Regorafenib was effective in prolonging PFS in advanced gastric adenocarcinoma in second- and third-line settings (2.6 versus 0.9 months, HR 0.40, p < 0.001) but no significant improvement in OS (5.8 versus 4.5 months, HR 0.74, p = 0.147). The most frequently seen side effects in all grades were fatigue (43.3%) and anorexia (36.1%). G3/4 toxicities with an incidence of ⩾5% of participants included hypertension (10.3%), anorexia (6.2%), rash (6.2%), abdominal pain (5.2%) and liver toxicities with elevated aspartate transaminase (AST; 9.3%), ALT (8.2%), GGT (6.2%). Hematological toxicity was uncommon.
Immune-checkpoint inhibitors (anti-programmed cell death protein 1 monoclonal antibodies)
Nivolumab
The ATTRACTION-2 study was a double-blind, placebo-controlled phase III RCT conducted in 49 clinic sites in Japan, South Korea and Taiwan. A total of 493 patients (advanced or metastatic gastric or gastroesophageal junction cancer, refractory to or intolerant of two or more previous chemotherapies, ECOG 0 or 1, life expectancy more than 3 months, and naïve to anti-programmed cell death protein (PD)-1 therapy or other therapeutic antibiotics and pharmacotherapies for the regulation of T-cells) were randomized to receive nivolumab (3 mg/kg intravenously every 2 weeks) or placebo. Patients with ongoing or previous autoimmune disease or interstitial lung disease, active diverticulitis or gastrointestinal ulcerative disease, or other uncontrolled or clinically significant medical disorders were not eligible for enrolment.
Nivolumab was associated with a significant OS benefit versus placebo (OS: 5.32 versus 4.14 months, p < 0.0001; 12-month OS: 26.6% versus 10.9%).21 Although the absolute gain in OS for nivolumab was only 1.1 months, it reduced the mortality risk by 37% compared with that of placebo. The survival advantage was persistent over time with nivolumab and irrespective of PD-1/ programmed death ligand (PD-L)1 expression. Nivolumab also significantly improved PFS versus placebo (PFS: 1.61 versus 1.45 months, p < 0.0001). The overall response rate in the nivolumab group was 11.2% and the median time to response was 1.6 months.
A treatment-related AE of any grade was reported in 43% patients receiving nivolumab and 27% patients in the placebo group. All grade TRAEs reported in 5% or more of patients in the nivolumab group were pruritus, diarrhea, rash and fatigue. Grade ⩾3 TRAEs occurred in 10% of patients in the nivolumab group versus 4% in the placebo group. The Grade ⩾3 TRAEs in the nivolumab group included interstitial lung disease, colitis, pyrexia, pneumonia, urinary tract infection and diabetic ketoacidosis.
Pembrolizumab
Pembrolizumab is another humanized anti-PD-1 monoclonal antibody. In the KEYNOTE-059 Cohort 1, a multicenter, open-label, single-arm phase II trial conducted at 67 sites in 17 countries, 259 patients (after failing two or more lines of chemotherapy including cisplatin and 5FU; patients with Her-2 positive tumors must have received treatment with trastuzumab) received a fixed dose of 200 mg pembrolizumab in a 3-weekly cycle.22 Patients with active autoimmune disease, immunodeficiency, receiving systemic steroid or any immunosuppressive therapies, prior anticancer monoclonal antibodies, known CNS metastasis, and hepatitis B/C were excluded.
Pembrolizumab showed an objective response rate of 11.6% (95% CI 8.0–16.1%), with complete response of 2.3% (95% CI 0.9–5.0%). The response rate was higher in the patients with PD-L1 positive tumors (PD-L1-positive versus PD-L1-negative: 15.5% versus 6.4%). A total of seven (4%) tumors were microsatellite instable (MSI)-high (H) and the response rates were higher, with an overall response rate of 57.1%. Median PFS was 2.0 months and median OS was 5.6 months. Based on this result, the United States Food and Drug Administration (US FDA) has approved pembrolizumab as the third-line treatment for PD-L1-positive gastric adenocarcinoma.
A TRAE of any grade was reported in 60.2% of patients receiving pembrolizumab. The most common any-grade AEs were fatigue, pruritis, rash, hypothyroidism, decreased appetite, anemia, nausea, diarrhea and arthralgia. Grade ⩾3 treatment-related AEs occurred in 17.8% patients, with more common AEs including anemia, fatigue and diarrhea. Overall, 17.8% of patients experienced at least one immune-mediated AE of any grade; the most common were hypothyroidism (8.9%), hyperthyroidism (3.5%) and colitis (2.3%).
Selecting a treatment
There is no consensus on the best regiment for metastatic gastric cancer in the third-line setting internationally. The National Comprehensive Cancer Network (NCCN) recommends TAS-102 in the third-line setting and pembrolizumab for the third-line or beyond treatment in adenocarcinoma of the stomach that expressed PD-L1 with a combined positive score (CPS) of greater than or equal to 1.23 The Japanese Gastric Cancer Guidelines suggests the use of nivolumab or irinotecan as a third-line treatment.24 Although guidelines are useful for the general population, it is always challenging to select the best treatment for each individual patient. Factors including baseline performance status, comorbidities, disease burden and response to previous therapies should be considered in selecting the most suitable regimen for the patient.
Table 2 outlines our recommendations for specific patient populations. As there is a lack of head-to-head comparisons, our recommendations rely on the subgroup analyses from pivotal studies, other prospective or retrospective studies and our own clinical experience.
Table 2.
Recommendations for specific patient populations.
| Characteristics | Taxane/irinotecan | TAS-102 | Apatinib | Regorafenib | Nivolumab | Pembrolizumab | |
|---|---|---|---|---|---|---|---|
| Ethnicity | - Asian |
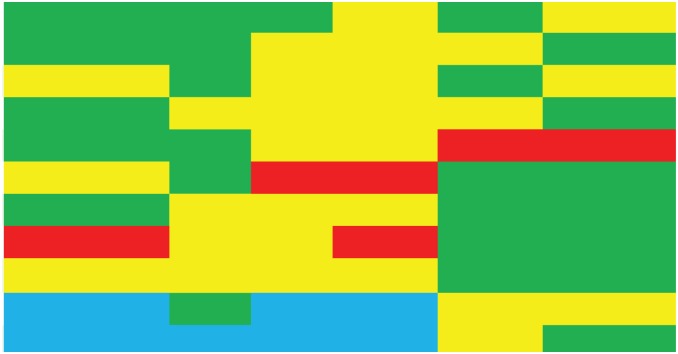
|
|||||
| - Non-Asian | |||||||
| High tumor burden | |||||||
| Peritoneal metastases | |||||||
| Autoimmune disease | |||||||
| Cardiovascular disease | |||||||
| Renal impairment* | |||||||
| Hepatic impairment* | |||||||
| Treatment line > 3 | |||||||
| Prior ramucirumab | |||||||
| PD-L1 +ve or MSI-H | |||||||
Green: Preferred options; Yellow: Alternatives; Blue: Insufficient data; Red: Used with caution.
Mild to moderate. No data for severe organ function impairment.
MSI-H, microsatellite instable high; PD-L1, programmed death ligand 1.
Irinotecan/taxane chemotherapy
In the landmark ‘salvage chemotherapy study’ by Kang and colleagues, subgroup analysis showed no significant benefit of palliative chemotherapy with irinotecan or docetaxel when used in a third-line setting.17 A Korean retrospective study with 158 patients using FOLFIRI as a third-line treatment showed a median PFS of 2.1 months and a median OS of 5.6 months with an objective response rate (ORR) of 9.6%. Independent prognostic factors related to prolonged OS and PFS were good PS (0/1), two or fewer metastatic sites and ⩾10.5 months from the first-line to third-line.25 Since there is only level II evidence to prove its efficacy, the use of irinotecan/taxane chemotherapy should be reserved if other options are not readily available.
TAS-102
TAS-102 should be considered as a preferred third-line option across the population of patients with metastatic gastric cancer. In the TAGS study, TAS-102 demonstrated a benefit versus BSC for both OS and PFS.18 The study included both Asian and western populations. It also included patients who used standard second-line therapies, including taxane, irinotecan and ramucirumab. Activity in the overall study population and subgroup analysis support the use of TAS-102 for patients with a heavy tumor burden (three or fewer metastatic sites, HR 0.71, 95% CI 0.54–0.94), Her-2 negative tumors (HR 0.62, 95% CI 0.48–0.82), no peritoneal metastasis (HR 0.66, 95% CI 0.51–0.86), previous use of taxane chemotherapy (HR 0.64, 95% CI 0.51–0.80), using TAS-102 after two lines of previous treatment rather than later lines (two versus three or more, p = 0.0014).
In the TAGS study, 58% of patients in the TAS-102 arm needed dosing modification (i.e. dosing delay or dose reduction) because of any-grade AEs of any cause). The high rate of dose modifications raised tolerability concerns, but most of those were mainly due to myelosuppression and gastrointestinal events, which were predictable and manageable by most oncologists. Neutropenia could be managed with dosing delays or administration of granulocyte colony-stimulating factor. TAS-102 has not been studied in patients with severe hepatic or renal impairment, and may be inappropriate for patients who required other immunosuppressive therapy.
Apatinib
Apatinib has shown an improvement of 1.8 months in median OS (HR 0.709) in a Chinese phase III placebo-controlled trial.19 It is now licensed in China as a third-line treatment. A global phase III trial is now ongoing to confirm whether the benefit can be recapitulated in the western population. Safety and tolerability data of apatinib were generally consistent with other VEGFR inhibitors with AEs including hypertension, proteinuria and hand-foot syndrome. Apatinib may be an option for Asian patients with ECOG 0, two or fewer metastatic sites, controlled hypertension, no bleeding tendency and not on thrombolytics or anticoagulants.
Regorafenib
Regorafenib could be considered as a preferred third-line option for metastatic gastric cancer. Regorafenib demonstrated PFS benefit versus BSC and no decrement in quality of life (QoL). The benefit was maintained in the subgroup analyses for patients aged ⩾60 years, more than two metastatic sites and the presence of peritoneal metastasis.20 Regorafenib was well tolerated with side effects mainly of hypertension, fatigue and hand-foot syndrome. There are no data on patients with severe renal or hepatic impairment. The efficacy of regorafenib is now being tested in a phase III trial (INTEGRATE II, ClinicalTrials.gov identifier: NCT02773524).
Nivolumab
Nivolumab showed an improvement in both OS and PFS.21 Complete remission was not achieved; however, the partial remission rate was 11%. Although the absolute benefits for OS and response rate were modest, this is promising in heavily treated patients after failing multiple lines of treatment. Activity in the overall study population and subgroup analysis support the use of TAS-102 for patients with a heavy tumor burden (two or more metastatic sites, HR 0.62, 95% CI 0.49–0.79) and previous ⩾4 lines of treatment. The survival benefit was independent of PD-L1 status. Caution should be taken when interpreting these results considering the small sample size of the PD-L1-positive subgroup. The ATTRACTION-2 study population were all Asian and whether the results are transferrable to the western populations is unclear.
Pembrolizumab
Pembrolizumab should be an attractive third-line option for tumors with PD-L1-positive or MSI-H tumors.22 Since KEYNOTE-059 was a single-arm cohort, further randomized trials are warranted to confirm its benefit.
Future directions
The gastric cancer treatment paradigm will continue to evolve as novel agents are developed and second or third-line agents are now being evaluated in the first-line settings. The World Health Organization classifies gastric cancer into papillary, tubular, mucinous and poorly cohesive carcinomas.26 The Lauren classification divides gastric cancer into intestinal, diffuse, and mixed types. However, these classifications do not give any predictive value when deciding the management of advanced gastric cancer.
The Cancer Genome Atlas research group categorized gastric cancer into four groups by comprehensive molecular characterization: Epstein–Barr virus (EBV) -positive, MSI, genome stable (GS) and chromosomal instable tumors (CIN).27 EBV-positive tumors (9% of cases) are characterized by EBV infection and showed extensive DNA promoter hypermethylation. They have the highest frequency of PIK3CA mutations (80%), and amplifications of JAK2 or PD-L1/L2 genes. The MSI subgroup (22% of cases) was characterized by genomic instability, due to a deficient DNA mismatch repair system, and lacked targetable amplifications. This subtype shows hypermethylation of the MLH1 promoter region and a very high mutation rate with hotspot mutations involving several genes like Her-2, Her-3, EGFR, JAK2, FGFR2, MET, and PIK3CA. In addition, MSI tumors have a high rate of PD-L1 expression which could make them very sensitive to checkpoint inhibitors. The GS subgroup (20% of cases) is related to CLDN18, RHOA, ARID1A mutations. In CIN tumors (50% of cases), genomic amplifications are frequent, with involvement of different tyrosine kinase receptors or related pathways.
Evidence showed that two subgroups of gastric cancer, as characterized by MSI-H and EBV-positive status, which may benefit from immunotherapy. Studies are currently underway to test pembrolizumab as first-line or second-line treatment for patients with advanced gastric cancer. Improving PFS in an earlier line of treatment would probably change the treatment strategies and improve the overall outcomes.
Tumor-specific biomarker stratification is likely to be relevant to targeted and immunotherapy approaches. However, it is not yet used for patient stratification or selection. Future studies are warranted to focus more on gene signaling to guide patient selection.
From KEYNOTE-059, ATTRACTION-2 and Javelin-30028 (which evaluated avelumab with chemotherapy), the response rates in these studies are all around 10%. In these studies, responses are observed in both PD-L1-positive and negative tumors, suggesting PD-L1 status is not a robust biomarker in gastric cancer. Another important note is the method of interpretation of PD-L1 positivity in different trials. KEYNOTE-059 counts tumor and stromal immune cells towards total PD-L1 positivity (CPS) while ATTRACTION-2 counts PD-L1 staining on tumor cells (i.e. tumor proportion score) only.21,22 This also accounts for the differences in PD-L1 dependence in KEYNOTE-059 and ATTRACTION-2. Future studies are warranted to focus on the biomarkers to separate responders and nonresponders to guide patient selection for immunotherapy.
Trials testing on the combination of immunotherapy with targeted agents or chemotherapy are ongoing. A phase I study with a combination of ramucirumab and durvalumab showed a promising response rate of 36% in patients with PD-L1 ⩾ 25%.29 The combination approach should be explored in the first-line to late-line treatment.
The majority of patients in the above trials did not receive ramucirumab in the second-line. Even in trials with the involvement of patients using ramucirumab in the second-line (TAS-102 and nivolumab), the OS was not significantly improved in the subgroup analysis. Further studies should be performed to have the optimal treatment sequencing for prolonging survival and preserving QoL.
Gastric cancer patients with progression after two lines of palliative systemic treatment usually have poor prognosis and short life expectancy. Endpoints on survival or PFS may not be the most suitable outcome. ‘Quality-adjusted survival’ or ‘time of preservation of functional capacity or independence’ might also be used as outcome measures in future research.
Conclusions
There is emerging evidence to support the efficacy of third-line systemic treatment in gastric cancer. RCTs with chemotherapy (TAS-102), targeted therapy (apatinib, regorafenib) and immunotherapy (nivolumab) all reported significant survival benefits. Future studies with a combination of chemotherapy, targeted agents and immunotherapy may help to maximize the benefits in both survival and QoL. Research on treatment-specific predictive biomarkers are warranted to identify optimal patients for third-line therapies.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Wing-lok Chan, Department of Clinical Oncology, Queen Mary Hospital, The University of Hong Kong, 1/F Professorial Block, 102 Pokfulam Road, Hong Kong.
Ka-on Lam, Department of Clinical Oncology, Queen Mary Hospital, The University of Hong Kong, 1/F Professorial Block, 102 Pokfulam Road, Hong Kong.
Tsz-him So, Department of Clinical Oncology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Victor Ho-fun Lee, Department of Clinical Oncology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Lai-wan Dora Kwong, Department of Clinical Oncology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
References
- 1. UICC. GLOBOCAN, www.uicc.org/new-global-cancer-data-globocan-2018 (2018, ).
- 2. Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol 2006; 12: 3237–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunderson LL. Gastric cancer—patterns of relapse after surgical resection. Semin Radiat Oncol 2002; 12: 150–161. [DOI] [PubMed] [Google Scholar]
- 4. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017; 8: CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 6. Kim HS, Kim HJ, Kim SY, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol 2013; 24: 2850–2854. [DOI] [PubMed] [Google Scholar]
- 7. Lacovelli R, Pietrantonio F, Farcomeni A, et al. Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer. A systematic review and meta-analysis of published studies. PLoS One 2014; 9: e108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liepa A, Mitchell S, Batson S, et al. Systematic review and meta-analysis of recommended second-line therapies for advanced gastric cancer (GC). In: European Cancer Congress Conference 2015, Vienna Austria: ECC, 2015. [Google Scholar]
- 9. Zhu X, Ko YJ, Berry S, et al. A Bayesian network meta-analysis on second-line systemic therapy in advanced gastric cancer. Gastric Cancer 2017; 20: 646–654. [DOI] [PubMed] [Google Scholar]
- 10. Harvey RC. Second-line treatments for advanced gastric cancer: a network meta-analysis of overall survival using parametric modelling methods. Oncol Ther 2017; 5: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badiani B, Maratea D, Messori A. Second-line treatments for advanced gastric cancer: interpreting outcomes by network meta-analysis. World J Clin Oncol 2015; 6: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koo DH, Ryu MH, Ryoo BY, et al. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer 2015; 18: 346–353. [DOI] [PubMed] [Google Scholar]
- 13. Choi IS, Choi M, Lee JH, et al. Treatment patterns and outcomes in patients with metastatic gastric cancer receiving third-line chemotherapy: a population-based outcomes study. PLoS One 2018; 13: e0198544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davidson M, Cafferkey C, Goode EF, et al. Survival in advanced esophagogastric adenocarcinoma improves with use of multiple lines of therapy: results from an analysis of more than 500 patients. Clin Colorectal Cancer 2018; 17: 223–230. [DOI] [PubMed] [Google Scholar]
- 15. Fanotto V, Uccello M, Pecora I, et al. Outcomes of advanced gastric cancer patients treated with at least three lines of systemic chemotherapy. Oncologist 2017; 22: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan WL, Yuen KK, Siu SW, et al. Third-line systemic treatment verse best supportive care for advanced/ metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017; 116: 68–81. [DOI] [PubMed] [Google Scholar]
- 17. Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. Clin Oncol 2012; 30: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 18. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018; 19: 1437–1448. doi: 10.1016/S1470-2045(18)30739-3 [DOI] [PubMed] [Google Scholar]
- 19. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016; 34: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 20. Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol 2016; 34: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 22. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NCCN. Clinical Practice Guidelines in Oncology. Gastric cancer version 2.2019, https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (2019, 03 June 2019).
- 24. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (version 4). Gastric Cancer 2017; 20: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang EJ, Im SA, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013; 16: 581–589. [DOI] [PubMed] [Google Scholar]
- 26. Lauwers GY, Caneiro F, Graham DY. Gastric carcinoma. In: Bowman FT, Carneiro F, Hruban RH. (eds) Classification of tumours of the digestive system. Lyon: IARC; 2010. [Google Scholar]
- 27. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase 3, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment for patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018; 29: 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bang YJ, Golan T, Lin CC, et al. Interim safety and clinical activity in patients with locally advanced and unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma from a multicohort phase I study of ramucirumab plus durvalumab. J Clin Oncol 2018; 36(Suppl 4S): abstr 92. [Google Scholar]


