Figure 1.
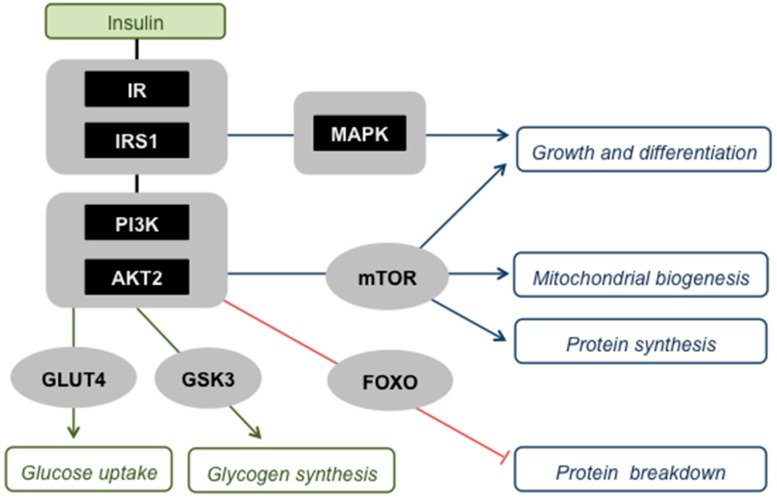
Skeletal muscle insulin signalling. Insulin activates its receptor (IR) and receptor substrate (IRS1), which comprise a ‘critical node’ in a branched signalling network that allows interaction with other pathways, for example those induced by cytokines [15]. Activation of this node triggers two major protein kinase cascades, i.e., the phosphatidylinositol-3-kinase (PI3K)—protein kinase B (AKT2 in skeletal muscle) pathway and the Ras-mitogen-activated protein kinase (MAPK) pathway, which both instruct muscle cells to engage with anabolic processes. Activated AKT2 has multiple effects: (i) It stimulates recruitment of the glucose transporter protein (GLUT4) to the plasma membrane, and is thus responsible for insulin-sensitive glucose uptake by muscle; (ii) it activates glycogen synthesis by inhibiting glycogen synthase kinase-3 (GSK3); (iii) it promotes mitochondrial biogenesis, protein synthesis, and cell growth and differentiation, effects that are all mediated through the stimulation of the mammalian target of rapamycin (mTOR); (iv) it suppresses protein breakdown by phosphorylating and thus deactivating Forkhead box O (FOXO) transcription factors that stimulate proteasome- and lysosome-mediated proteolysis. The MAPK pathway acts in concert with AKT2 to transmit insulin’s message to increase cell growth and differentiation.