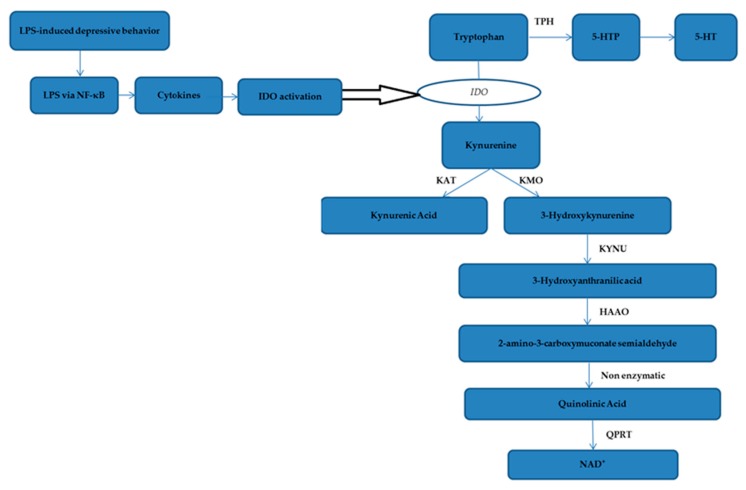
Figure 7.
IDO pathway and tryptophan metabolism pathway. Notes: Tryptophan catabolism through the kynurenine pathway involves IDO as an intracellular rate–limiting enzyme. Pro-inflammatory cytokine is upregulated by LPS regulated by NF-κB. Activation of NF-κB results in the release of pro-inflammatory cytokines, which in turn, activates IDO activity. Tryptophan may be metabolized to serotonin or alternatively, is metabolized via the kynurenine pathway. Tryptophan is metabolized to kynurenine by indoleamine 2,3-dioxygenase. Kynurenine is then converted via kynurenine aminotransferases (KAT) to kynurenic acid, a neuroprotective molecule as it antagonizes glutamate receptor-induced neurotoxicity. In addition, kynurenine can also be converted to 3-hydroxykynurenine by kynurenine-3-monooxygenase (KMO), for which evidence is accumulating of its neurotoxic capability. Sequential conversion to 2-amino-3-carboxymuconate-semialdehyde is the penultimate step leading to enzymatic production of (neuroprotective) picolinic acid, and the (non-enzymatic) production of the well-known neurotoxic compound quinolinic acid. Further conversion of QUIN to the essential cofactor NAD+ is catalysed by quinolinate phosphoribosyltransferase (QPRT).